Hot Paper
Eduard O. Bobylev, Julian Ruijter, David A. Poole III, Simon Mathew, Bas de Bruin, Joost N. H. Reek
Angewandte Chemie, International Edition, 2023, 62(16), e202218162
DOI: 110.1002/anie.202218162
Graphical Abstract
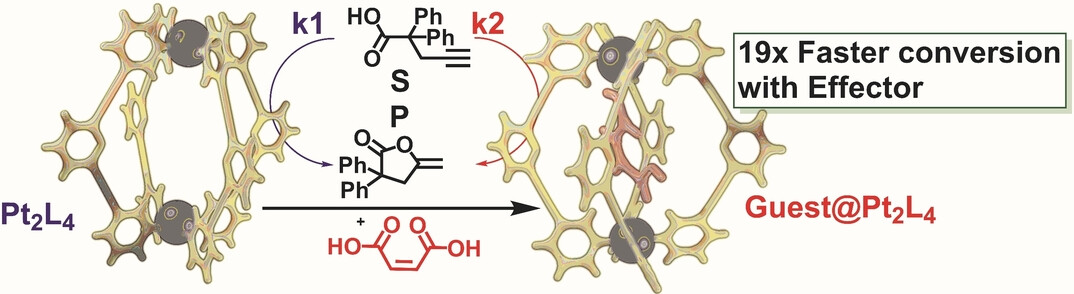
Pt2L4 cages are found to catalyze the cyclization of alkynoic acids. The reaction rates are depending on effector guests bound in the cage, leading to an enhancement of the catalytic rate by up to 19-fold or a decrease by 5-fold. In contrast to this effector-controlled rate enhancement using the binding pocket of Pt2L4, the molecular analogue PtL4 displays no significant changes in catalytic rate. By effector induced rate changes of cage catalyzed reactions, unexpected new reactivity and complex autoregulation comes in sight.
Abstract
Metabolic pathways are highly regulated by effector molecules that influences the rate of enzymatic reactions. Inspired by the catalytic regulation found in living cells, we report a Pt2L4 cage of which the activity can be controlled by effectors that bind inside the cage. The cage shows catalytic activity in the lactonization of alkynoic acids, with the reaction rates dependent on the effector guest bound in the cage. Some effector guests enhance the rate of the lactonization by up to 19-fold, whereas one decreases it by 5-fold. When mixtures of specific substrates are used, both starting materials and products act as guests for the Pt2L4 cage, enhancing its catalytic activity for one substrate while reducing its activity for the other. The reported regulatory behavior obtained by the addition of effector molecules paves the way to the development of more complex, metabolic-like catalyst systems.