Catalysis with earth abundant metals
A continuous need to develop new catalysts
The world is in continuous need of various new chemicals and energy. Several health issues related to food, modern lifestyle and aging call for the development of new or improved drugs, food ingredients and personal care ingredients. Furthermore, the production of enough food for our rapidly growing world population makes it crucially important to grow crops in the most efficient manner possible. This, in turn, requires the development of new, safe and bio-selective insecticides and fungicides, that are harmless to other organisms. Also the development of new antibiotics is of key importance, as many bacteria are developing resistance to the existing ones. Clearly, efficient and clean synthesis of new chemicals is essential. Synthetic chemistry has matured to such an advanced level that almost every desired molecule can be synthesized. However, the field is still subject to many stoichiometric transformations, which applies to many fine-chemical production plants and small-to medium-scale laboratories. Many of the presently known chemicals are prepared using complex, multistep procedures requiring several work-up steps and protecting group strategies. Catalysis offers numerous opportunities to solve such problems, allowing the preparation of important chemicals in cheaper, efficient and less labor-intensive and time-consuming manners. Hence, from a sustainability and energy-conservation point of view, developing efficient shortcuts in organic synthesis by means of catalysis is a highly desirable goal, especially considering the rapidly growing world population. There remains plenty of room for improvement in catalysis research, in many aspects and for several important goals. For instance, contemporary efforts which are ongoing globally to replace fossil-based resources by renewables (i.e. biomass) require the development of new catalysts that are more tolerant to water and a wide range of functional groups. Elegant chemo-, regio‑, diastereo- and enantioselective catalytic reactions are continuously being developed, but the development of highly selective catalysis has by no means matured to the required level needed for a truly sustainable society and for medicinal purposes. Furthermore, many of the known reactions have a limited substrate scope or require the development of a dedicated catalyst. As such, the development of efficient and selective catalysts remains one of the major research goals in modern chemistry. New catalysts are needed to arrive at selective and efficient methods for the preparation of a variety of organic materials, medicines and other bioactive compounds. At the same time, an increasing demand for sustainable energy and finding ways to store this energy in the form of chemical energy (fuels) puts high demands on the development of new electrocatalysts and photocatalysts, which should be stable, cheap, fast and durable. All these are goals are not easy to reach and require substantial efforts and profound fundamental research.
Moving from noble metals to base metals
Material scarcity is a particularly urgent issue to be addressed in modern catalysis research. With a rapidly increasing world population combined with higher living standards globally, many of our natural resources and in particular the scarce elements are depleting. Moving from fossil resources to renewables puts demands on the development of new catalysts, but also existing ones currently used extensively in industry may need to be replaced. With so many people driving a car, each equipped with e.g.car exhaust catalysts, noble metals like Pd, Pt and Rh are draining and hence become increasingly expensive. Noble metals are frequently applied in a variety of catalytic synthetic methodologies and many industrial processes rely on them. As such there is an increasing demand to replace expensive, non-abundant noble metals such as Rh, Ir, Pt and Ru (2ndand 3rdrow transition metals) by the considerably cheaper, earth-abundant (1strow) transition metals such as Mn, Fe, Co, Ni or Cu. This is not an easy task, as the reactivity of base metals is typically very different from that of noble metals. As such, many attempts to replace expensive, scarce and often toxic metals by much cheaper, abundant and more benign ones have been hindered by an entirely different chemical behavior of these metals.
The catalytic activity of noble metals most frequently involves well-established two-electron elementary steps such as oxidative addition, reductive elimination and β-hydrogen elimination. These elementary steps occur particularly easily for late 2ndand 3rdrow transition metals, as these predominantly occur in two stable oxidation states differing by two electrons (e.g. 0 and +II). Base metals (Mn, Fe, Co, Ni and Cu) on the other hand, have weaker metal-ligand bonds, often have a rather small energy separation between their valence orbitals, easily switch between low-spin and high-spin configurations (with associated coordination geometries and lability), and as a result have a much stronger tendency to react via one-electron (viz. radical) elementary steps (with the exception of less common low-spin diamagnetic complexes capable of two-electron substrate activation). As such, many researchers have attempted to make first-row transition metals behave as their second- or third row congeners in terms of reactivity towards typical organic substrates. Three main strategies to make efficient use of base metals in organometallic catalysis have emerged (see Scheme below). The strong-field approach is used to force integer spin complexes to adopt a low-spin configuration, often leading to diamagnetic systems with an increased tendency to facilitate two-electron elementary steps (‘noble metal behavior’).
A second approach is to make use of redox-active ligands, which are ligands that can store and release electrons to the metal and/or coordinated substrate ligands. In this way, some (or sometimes all) of the electrons required for substrate activation stems from the ligand(s) rather than from the metal center, thus enabling two-electron processes to occur also for base metals.
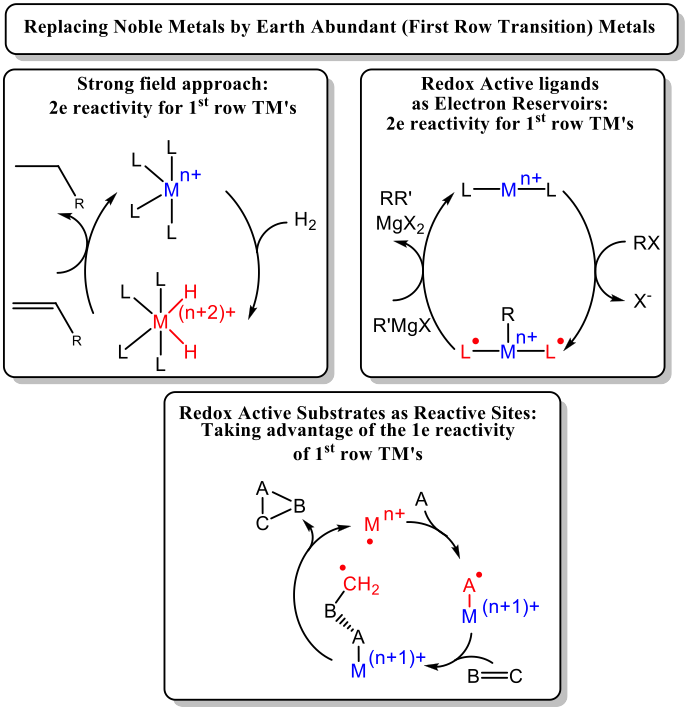

The third strategy, which is currently becoming more popular, actually takes advantage of the one-electron reactivity of base metals. This approach makes use of the redox-active behavior of certain coordinated substrates, leading to metal-to-substrate or substrate-to-metal electron transfer, thus forming substrate-centered radicals that can be used in catalytic radical-type transformations. This type of mechanism bears a resemblance to that of radical-type reactions catalyzed by (transition metal based) metalloenzymes, and as such one could call this approach ‘bio-inspired’. This approach has the additional advantage that it also leads to novel open-shell (radical-type) reactivity, inaccessible to traditional noble metal catalysis, and hence is also of profound fundamental interest.
Redox noninnocent ligands in catalysis
Besides acting as electron reservoirs, redox active/noninnocent ligands can actually play many more roles in catalysis. They facilitate low-barrier (radical) pathways in controlled catalysis with earth abundant metals in the coordination sphere of transition metals and stabilize reactive intermediates.
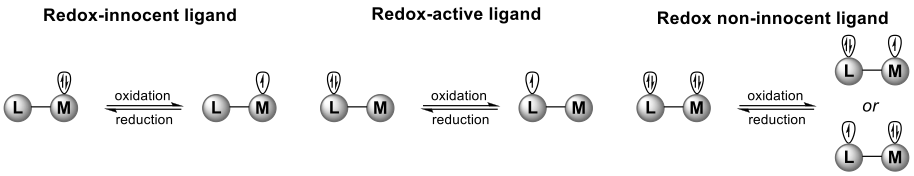
In the Homkat group we are investigating, in detail, the various roles redox active ligands and redox noninnocent ligands can play in catalysis and how these ligands can be used to control catalysis with first row transition metals. Redox active/noninnocent ligands can aid catalytic (radical-type) reactivity by amongst others: (i) changing the Lewis acidity or basicity of the metal center, (ii) acting as an electron reservoir, (iii) generating a reactive ligand-centered radical, (iv) assisting spin flip events, and (v) activation of a substrate in a radical-type manner.
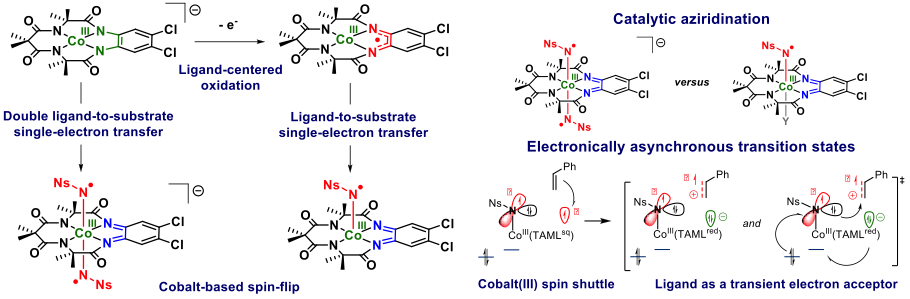
Some illustrative examples of recently uncovered and unexpected electron transfer, spin-flips and electronically asynchronous transition states made possible by a redox active TAML ligand are shown in the figure above.
