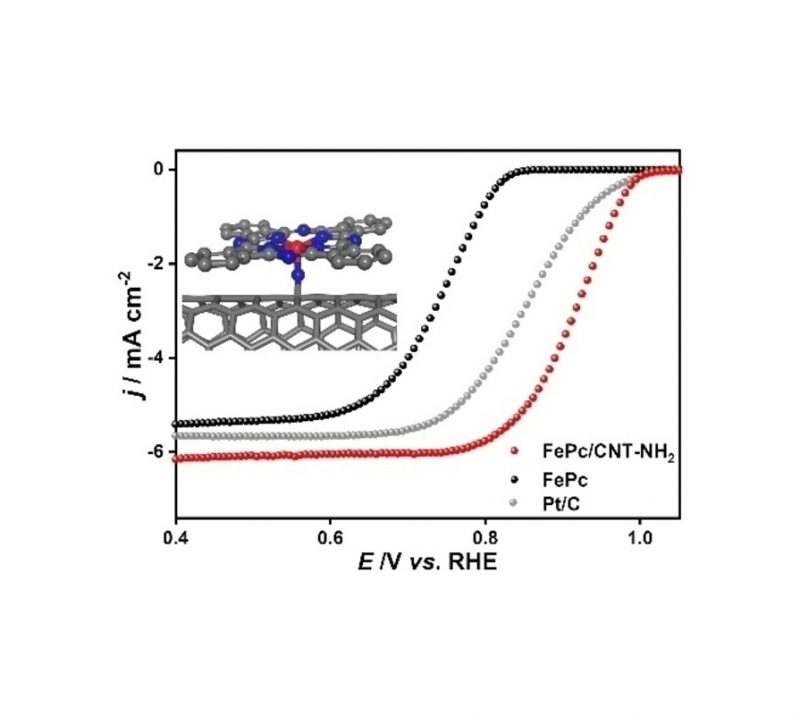
Soumyadeep Chakrabortty, Katharina Konieczny, Felix J. de Zwart, Eduard. O. Bobylev, Eszter Baráth, Sergey Tin, Bernd H. Müller, Joost N. H. Reek, Bas de Bruin, and Johannes G. de Vries
Angew. Chem. Int. Ed., 2023, 62(26), e202301329
DOI: 10.1002/anie.202301329

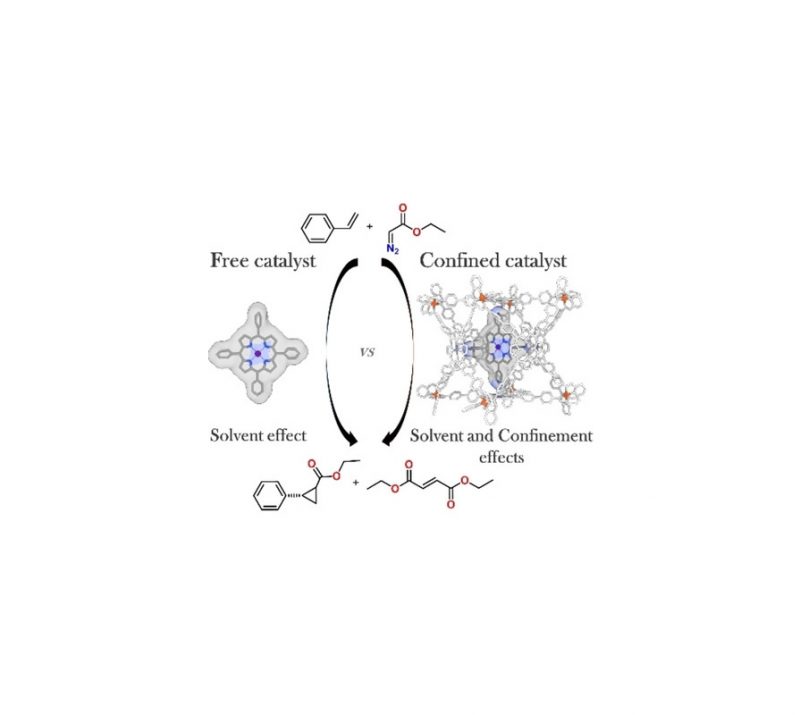
Graphical Abstract
The synthesis of chiral cyclic amides has been achieved via cobalt-catalyzed asymmetric hydrogenation of the corresponding enamides with a high degree of enantioselectivity. This methodology extends the utilization of earth abundant metal catalysis in asymmetric synthesis.
Abstract
The enantioselective hydrogenation of cyclic enamides has been achieved using an earth-abundant cobalt-bisphosphine catalyst. Using CoCl2/(S,S)-Ph-BPE, several trisubstituted carbocyclic enamides were reduced with high activity and excellent enantioselectivity (up to 99 %) to the corresponding saturated amides. The methodology can be extended to the synthesis of chiral amines by base hydrolysis of the hydrogenation products. Preliminary mechanistic investigations reveal the presence of a high spin cobalt (II) species in the catalytic cycle. We propose that the hydrogenation of the carbon-carbon double bond proceeds via a sigma-bond-metathesis pathway.