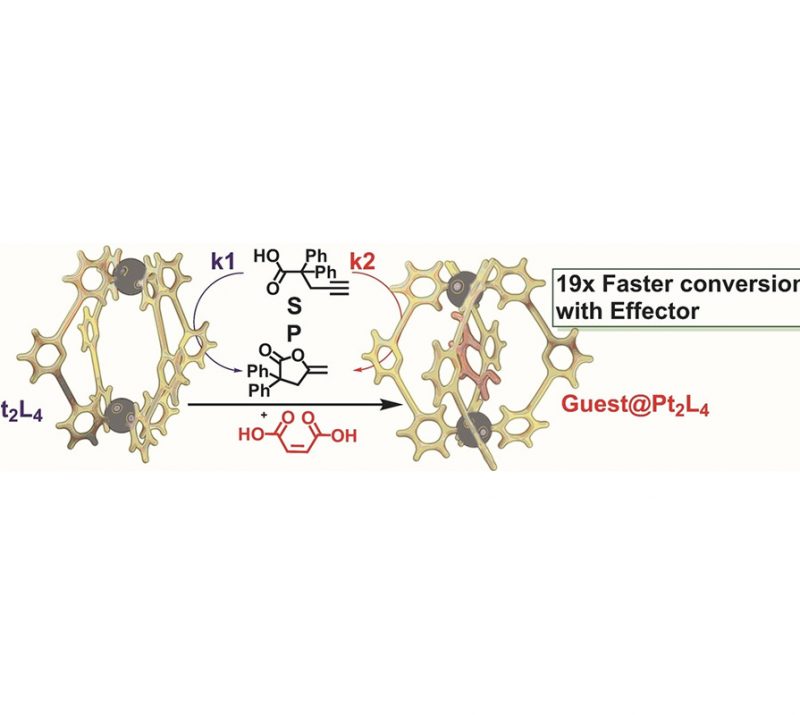
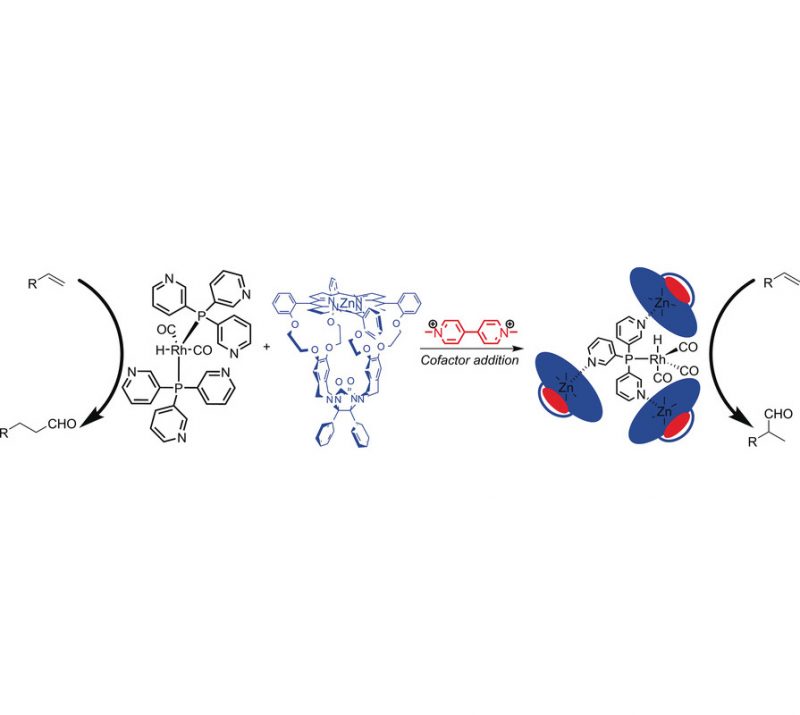
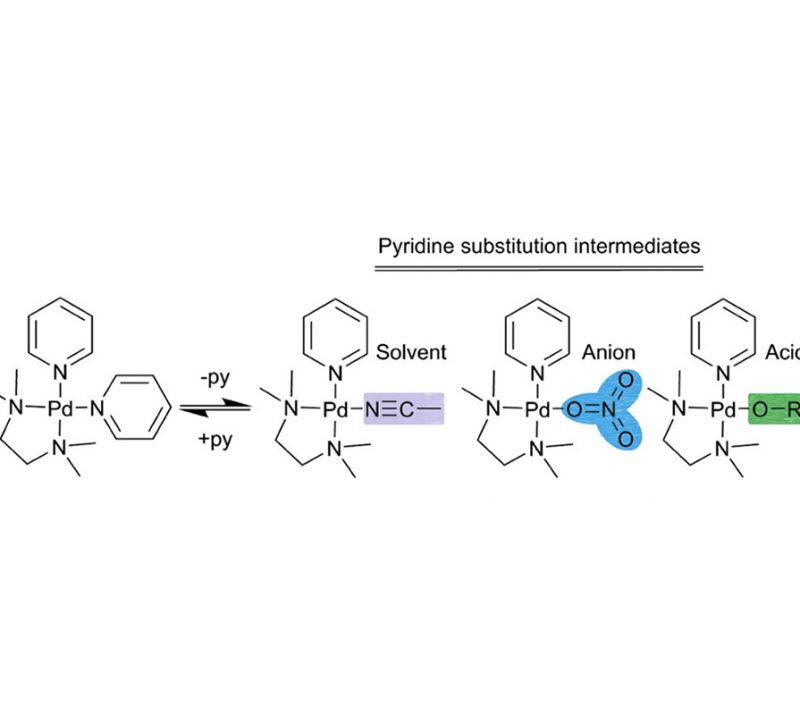
Very Important Paper
Lu‐Hua Zhang, Simon Mathew, Joeri Hessels, Joost N. H. Reek, Fengshou Yu
ChemSusChem, 2021, 14(1), 234-250
DOI: 10.1002/cssc.202001876
Cover
Lu‐Hua Zhang, Simon Mathew, Joeri Hessels, Joost N. H. Reek, Fengshou Yu
ChemSusChem, 2021, 14(1), 2
DOI: 10.1002/cssc.202002769
Abstract
Strategies that enable the renewable production of storable fuels (i. e. hydrogen or hydrocarbons) through electrocatalysis continue to generate interest in the scientific community. Of central importance to this pursuit is obtaining the requisite chemical (H+) and electronic (e−) inputs for fuel‐forming reduction reactions, which can be met sustainably by water oxidation catalysis. Further possibility exists to couple these redox transformations to renewable energy sources (i. e. solar), thus creating a carbon neutral solution for long‐term energy storage. Nature uses a Mn−Ca cluster for water oxidation catalysis via multiple proton‐coupled electron‐transfers (PCETs) with a photogenerated bias to perform this process with TOF 100∼300 s−1. Synthetic molecular catalysts that efficiently perform this conversion commonly utilize rare metals (e. g., Ru, Ir), whose low abundance are associated to higher costs and scalability limitations. Inspired by nature‘s use of 1st row transition metal (TM) complexes for water oxidation catalysts (WOCs), attempts to use these abundant metals have been intensively explored but met with limited success. The smaller atomic size of 1st row TM ions lowers its ability to accommodate the oxidative equivalents required in the 4e−/4H+ water oxidation catalysis process, unlike noble metal catalysts that perform single‐site electrocatalysis at lower overpotentials (η). Overcoming the limitations of 1st row TMs requires developing molecular catalysts that exploit biomimetic phenomena – multiple‐metal redox‐cooperativity, PCET and second‐sphere interactions – to lower the overpotential, preorganize substrates and maintain stability. Thus, the ultimate goal of developing efficient, robust and scalable WOCs remains a challenge. This Review provides a summary of previous research works highlighting 1st row TM‐based homogeneous WOCs, catalytic mechanisms, followed by strategies for catalytic activity improvements, before closing with a future outlook for this field.