Catriona C. James, Petrus C. M. Laan, Bas de Bruin, Joost N. H. Reek
ChemCatChem, 2023, 15(7), e202201272
DOI: 10.1002/cctc.202201272
Graphical Abstract
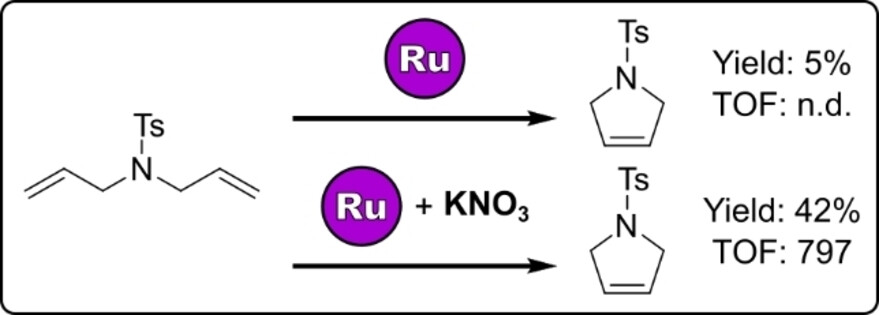
Kinetic protection of a transition metal catalyst: Addition of nitrate salt can bestow kinetic protection upon an olefin metathesis catalyst. In the presence of nitrate the catalyst exhibits much faster reaction kinetics, which allows productive catalysis reactions to occur faster than catalyst decomposition pathways.
Abstract
Olefin metathesis catalysts like AquaMet are vulnerable to different decomposition pathways under biologically relevant conditions. Currently, stabilizing strategies are focused on approaches with limited relevance for application under biologically relevant conditions. Initial attempts to stabilise AquaMet by encapsulation within a supramolecular metallocage showed that the nitrate counterions of the cage improve the activity of the catalyst. We show that the chloride ligands of AquaMet can be replaced with nitrates by simple anion-exchange. Catalytic studies into metathesis of a diallyl substrate showed that the presence of nitrate generates higher yields of the ring-closed product compared to AquaMet alone, under aqueous and biological conditions. Kinetic studies support that the nitrate-containing catalyst both initiates faster and performs catalysis at a much faster rate than AquaMet, while the rate of catalyst deactivation was similar. This new strategy of kinetic protection of a transition metal catalyst may have future applications for other catalytic reactions applied in vivo.