Metalloradical Catalysis
Bio-inspired controlled radical reactivity in organometallic catalysis
In organometallic chemistry one-electron and radical-type elementary steps remain relatively underexplored in the development of new catalytic reactions. Radicals (and related hypovalent reagents) are intrinsically reactive, and radical-type reactions are often associated with uncontrolled, non-selective processes such as those observed in free-radical chain-reactions (free-radical polymerization, radical halogenation, auto-oxidation, etc.) and the undesired free-radical processes that are responsible for e.g. DNA damage, cancer, aging and cell-death. As a result, radicals were long believed to be too reactive to be selective. This is, however, a dogma and in fact a proven misconception.Selectivity is a matter of relative reactivity, and as such controlled radical reactions are certainly feasible. Free radicalsindeed react ‘randomly’ and are difficult to control, therefore leading to the above mentioned biological problems and poor selectivity in synthetic reactions. However, in the coordination sphere of a transition metal, radical processes can be controlled with ultrahigh precision. In fact, Nature solves its most difficult bio-synthetic problems using controlled radical-type reactions catalyzed by metalloenzymes. Despite their radical character, these reactions proceed with high chemo-, regio‑, diastereo and enantioselectivity. As such, metalloenzymes form an important source of inspiration for the development of new catalytic reactivity on the basis of radical-type elementary steps. In the Homkat group we are pioneeringmetalloradical organometallic catalysis research.
Carbene & nitrene radical chemistry
In the Homkat group we developed several new reactions based on the one-electron radical-type reactivity of cobalt(II) in reactions with carbene- and nitrene precursors (see scheme below). The mechanisms of these reactions have been studied in detail using a combination of spectroscopic studies, model reactions, spin trapping and supporting DFT calculations. These studies clearly revealed the redox noninnocence of nitrenes and carbenes when bound to cobalt(II), which convert into cobalt(III) nitrene- and carbene radical species (one-electron reduced nitrene and carbene units) by intramolecular single-electron transfer from cobalt(II) (or other metalloradicals) to the nitrene or carbene moiety (see Figure below). As such, discrete nitrogen- or carbon centered radical species are generated, and these ‘carbene-radicals’ and ‘nitrene radicals’ can mediate a variety of selective radical-type transformations.
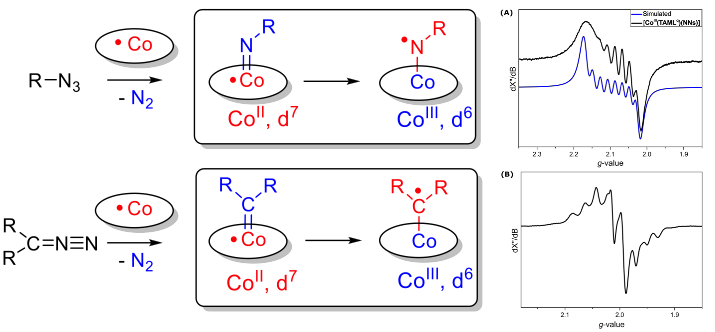
Discrete spin density at the carbon and nitrogen atoms of the abovementioned nitrene- and carbene radical species leads to typical radical-type reactivity, distinct from traditional closed-shell (Fischer-type) nitrenes and carbenes.
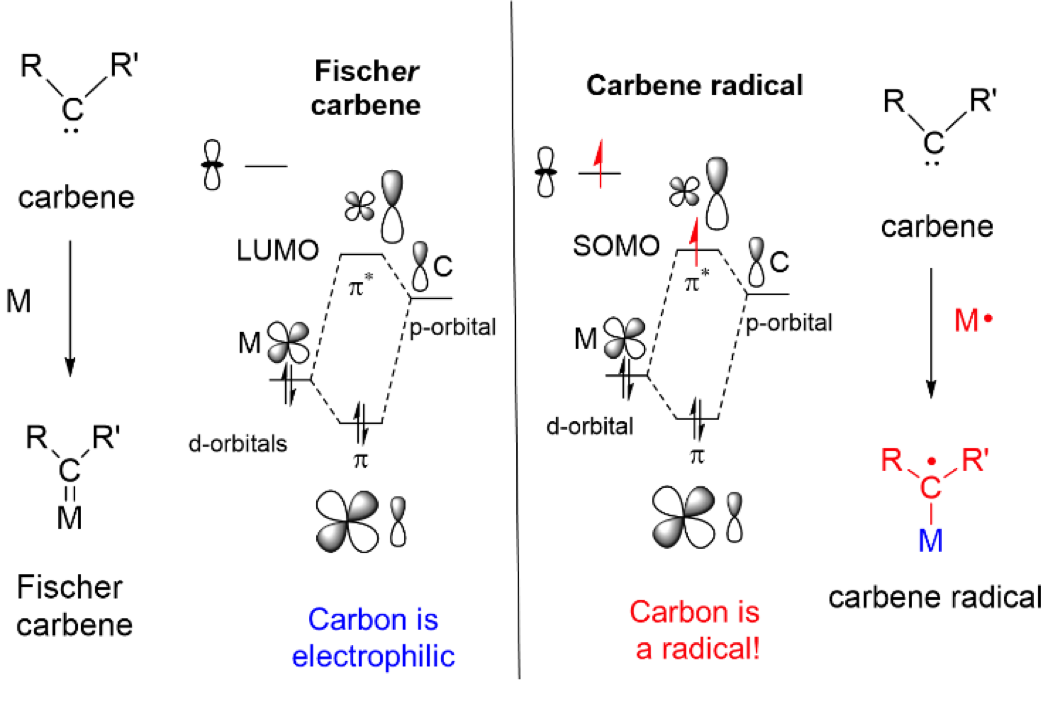
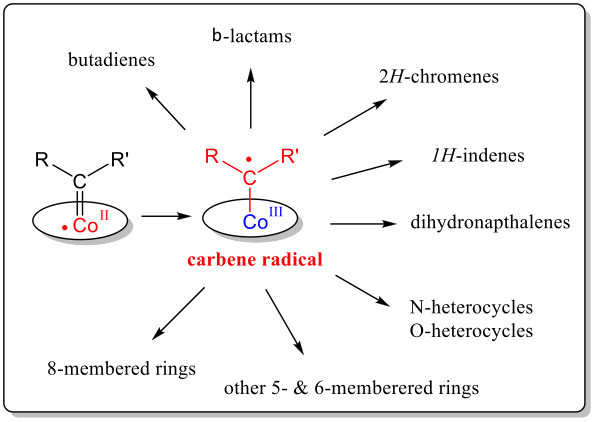
The radical-type reactivity of carbene radical species can be easily understood on the basis of their frontier orbital interactions (see Figure above). Low-lying carbene-dominated p*-antibonding orbitals facilitate intramolecular single-electron transfer from the metal to the carbene. This effectively produce a carbene-radical complex (one-electron reduced Fischer-type carbene/nitrene). The orbital arrangement in ‘nitrene radical’ species is similar. This leads to discrete substrate centered radical reactivity. Several new catalytic reactions are being developed in the Homkat group using the concept of metalloradical catalysis via carbene and nitrene radicals. Some illustrative examples are shown in the figures below.
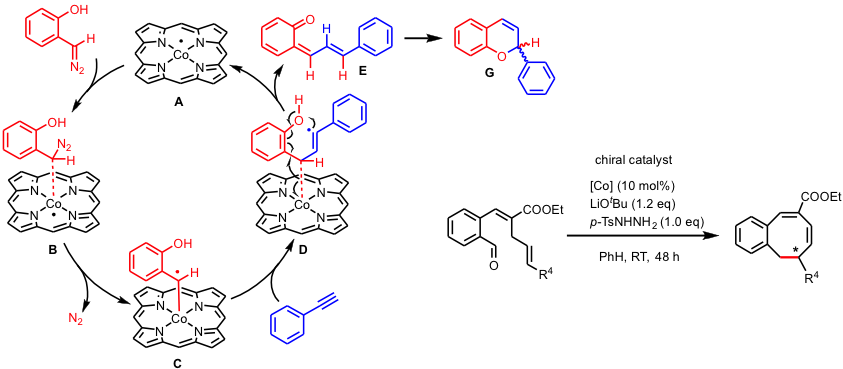
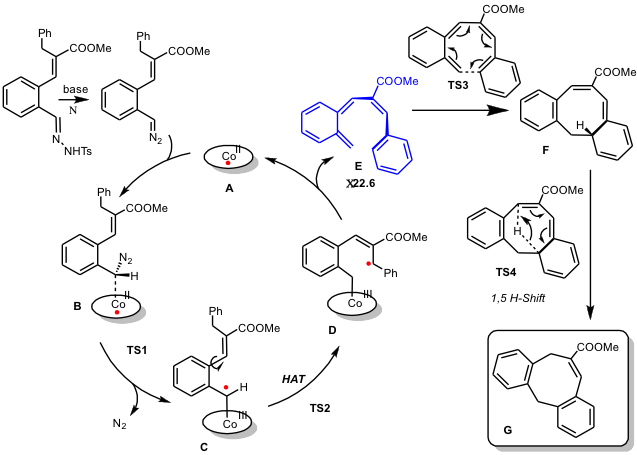
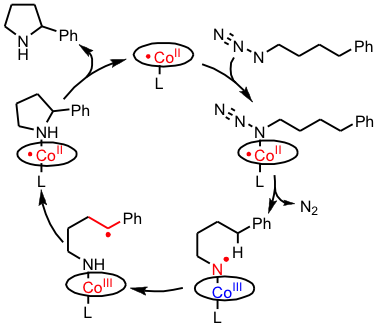
An example of the involvement of nitrene radical intermediates in catalytic ring-closure reactions of aliphatic azides to generateN‑heterocycles is shown below. Activation of the azide generates a discrete nitrene radical intermediate, with > 80% spin density on the nitrene nitrogen atom. Hydrogen atom abstraction followed by radical rebound results in catalytic formation of heterocycles.