Julien Daubignard, Remko J. Detz, Bas de Bruin, Joost N. H. Reek
Organometallics, 2019, 38(20), 3961-3969
DOI: 10.1021/acs.organomet.9b00484
Abstract
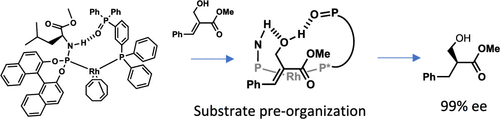
A series of bisphosphine monoxides and a phosphoramidite have been used for the preparation of supramolecular ligands. A structural analysis of the complexes using NMR spectroscopy and DFT calculations revealed the formation of strong hydrogen bonding between the two ligands. The complexes have been evaluated in the hydrogenation of several functionalized alkenes, leading to very high enantioselectivity for the substrates bearing a hydroxyl group. Also, kinetic studies showed that enhanced reaction rates of hydrogenation are observed in comparison with the supramolecular catalytic systems based on urea groups. In-depth NMR spectroscopy experiments and computational studies have highlighted the crucial role of the hydrogen bond between the phosphine oxide ligands and the substrate during the hydrogenation reaction.