Xander Schaapkens, Joost N. H. Reek, Tiddo J. Mooibroek
Inorganic Chemistry Communications, 2022, 139, 109284
DOI: 10.1016/j.inoche.2022.109284
Abstract
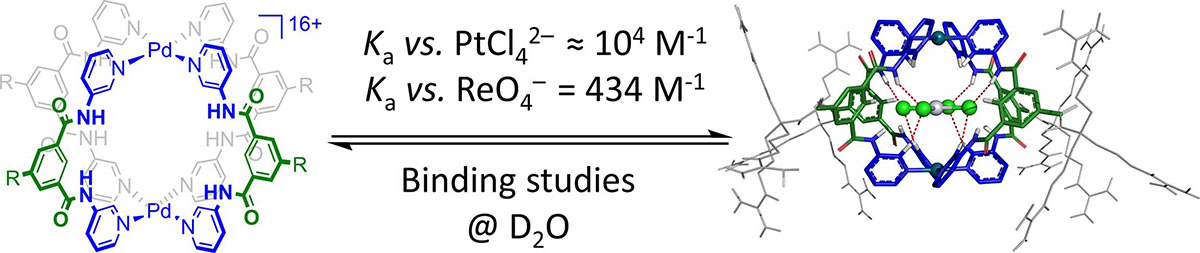
Anions such as pertechnetate (99mTcO4–) and tetrachloroplatinate (195mPtCl42–) are important precursors for radioactive substances for medical imaging. Molecules that can sense and bind these small anions in water can be exploited to circumvent lengthy synthesis on radioactive material and improve these applications. The most important property of a host is its selectivity for non-biological anions over common biological anions and/or molecules. Here, we report that a previous published [Pd2L4]16+ guanidinium cage has higher selectivity for ReO4– and particularly for PtCl42– (nonradioactive substitutes for 99mTcO4– and 195mPtCl42–) over common biological molecules. This selectivity was shown by means of 1H NMR titrations with the most noticeable affinity for PtCl42– in the order of Ka = 104 M−1. A molecular model of PtCl42– or ReO4–bound inside the cage revealed the best interaction complementary fit with the PtCl42– anion, which is held in place by equally short N–H···Cl and C–H···Cl hydrogen bonds. This research shows that a simple coordination cage can already have selective binding for non-biological anions such as ReO4– and particularly for PtCl42–, paving the way to improve coordination cages for use in medical imaging research.