Raoul Plessius, Vera Deij, Joost Reek, Jarl Ivar van der Vlugt
Chem. Eur. J., 2020, 26, 13241 – 13248
Abstract
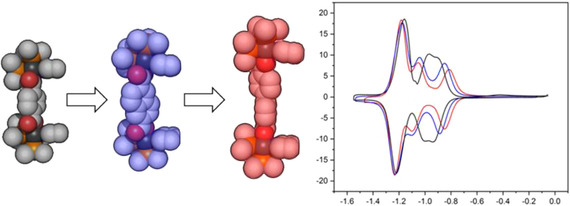
Three Pt4L2L′2 heteroleptic rectangles (1–3), containing ditopic redox‐active bis‐pyridine functionalized perylene bisimide (PBI) ligands PBI‐pyr2 (L) are reported. Co‐ligand L′ is a dicarboxylate spacer of varying length, leading to modified overall size of the assemblies. 1H NMR spectroscopy reveals a trend in the splitting and upfield chemical shift of the PBI‐hydrogens in the rectangles with respect to free PBI, most pronounced with the largest strut length (3) and least with the smallest strut length (1). This is attributed to increased rotational freedom of the PBI‐pyr2 ligand over its longitudinal axis (Npy‐Npy), due to increased distance between the PBI‐surfaces, which is corroborated by VT‐NMR measurements and DFT calculations. The intramolecular motion entails desymmetrization of the two PBI‐ligands, in line with cyclic voltammetry (CV) data. The first (overall two‐electron) reduction event and re‐oxidation for 1 display a subtle peak‐to‐peak splitting of 60 mV, whilst increased splitting of this event is observed for 2 and 3. The binding of pyrene in 1 is probed to establish proof of concept of host‐guest chemistry enabled by the two PBI‐motifs. Fitting the binding curve obtained by 1H NMR titration with a 1:1 complex formation model led to a binding constant of 964±55 m−1. Pyrene binding is shown to directly influence the redox‐chemistry of 1, resulting in a cathodic and anodic shift of approximately 46 mV on the first and second reduction event, respectively.