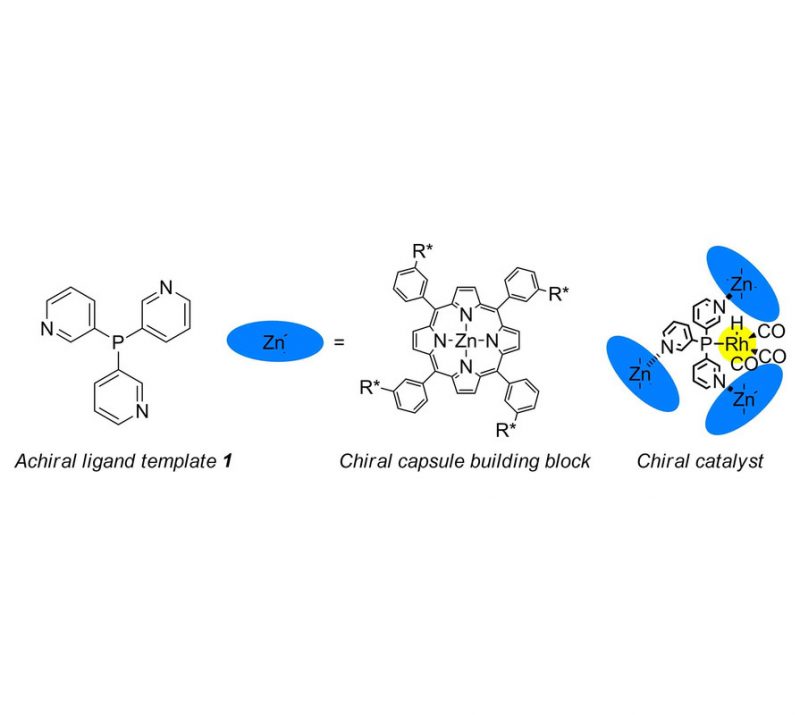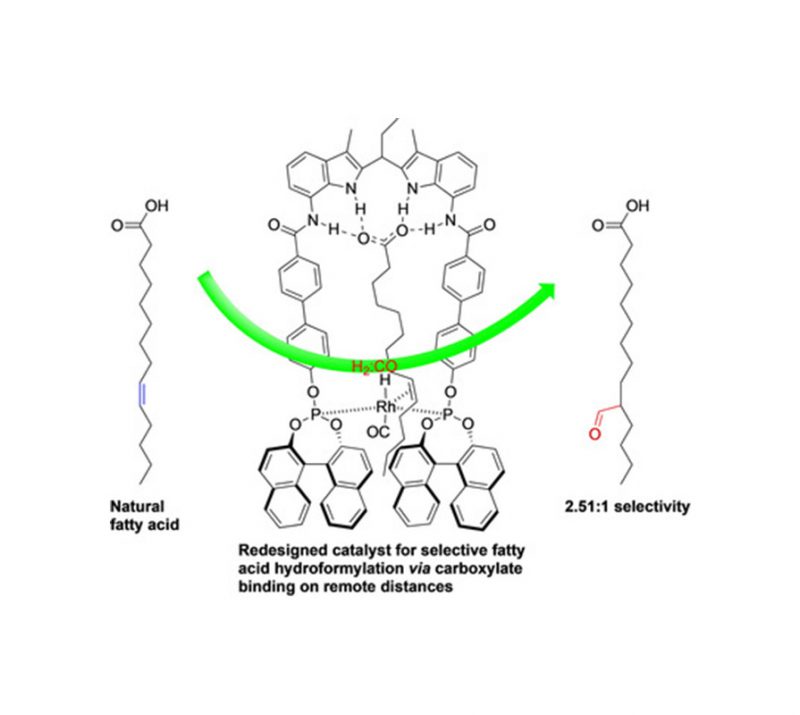
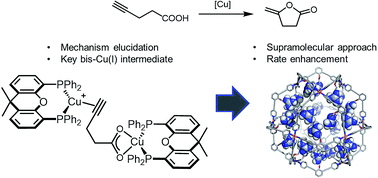
Sergio Gonell, Xavier Caumes, Nicole Orth, Ivana Ivanovic-Burmazovic, Joost N. H. Reek
Chemical Science, 2019, 10(5), 1316-1321
DOI: 10.1039/C8SC03767A
Abstract
The application of large M12L24 nanospheres allows the pre-concentration of catalysts to reach high local concentrations, facilitating reactions that proceed through dinuclear mechanisms. The mechanism of the copper(I)-catalyzed cyclization of 4-pentynoic acid has been elucidated by means of a detailed mechanistic study. The kinetics of the reaction show a higher order in copper, indicating the formation of a bis-Cu intermediate as the key rate determining step of the reaction. This intermediate was further identified during catalysis by CIS-HRMS analysis of the reaction mixture. Based on the mechanistic findings, an M12L24 nanosphere was applied that can bind up to 12 copper catalysts by hydrogen bonding. This pre-organization of copper catalysts in the nanosphere results in a high local concentration of copper leading to higher reaction rates and turnover numbers as the dinuclear pathway is favored.