Didjay F. Bruggeman, Tijmen M. A. Bakker, Simon Mathew, Joost N. H. Reek
Chem. Eur. J., 2021, 27(1), 218-221
DOI: 10.1002/chem.202003306
Abstract
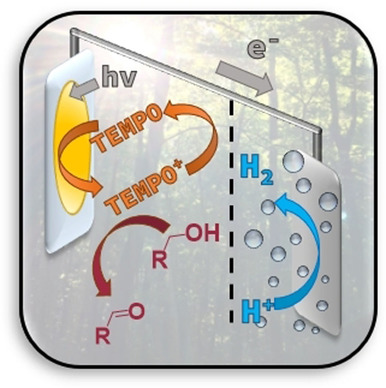
This work reports a dye‐sensitized photoelectrochemical cell (DSPEC) that couples redox‐mediated light‐driven oxidative organic transformations to reductive hydrogen (H2) formation. The DSPEC photoanode consists of a mesoporous anatase TiO2 film on FTO (fluorine‐doped tin oxide), sensitized with the thienopyrroledione‐based dye AP11, while H2 was formed at a FTO‐Pt cathode. Irradiation of the dye‐sensitized photoanode transforms 2,2,6,6‐tetramethylpiperidine 1‐oxyl (TEMPO) to the oxidized TEMPO (TEMPO+), which acts as a chemical oxidant for the conversion of benzyl alcohol. The TEMPO0/+ couple, previously used as redox mediator in DSSC, mediates efficient electron transfer from the organic substrate to the photo‐oxidized dye. A DSPEC photoreactor was designed that allows in situ monitoring the reaction progress by infrared spectroscopy and gas chromatography. Sustained light‐driven oxidation of benzyl alcohol to benzaldehyde within the DSPEC photoreactor, using of TEMPO as mediator, demonstrated the efficiency of the device, with a photocurrent of 0.4 mA cm−2, approaching quantitative Faradaic efficiency and exhibiting excellent device stability.