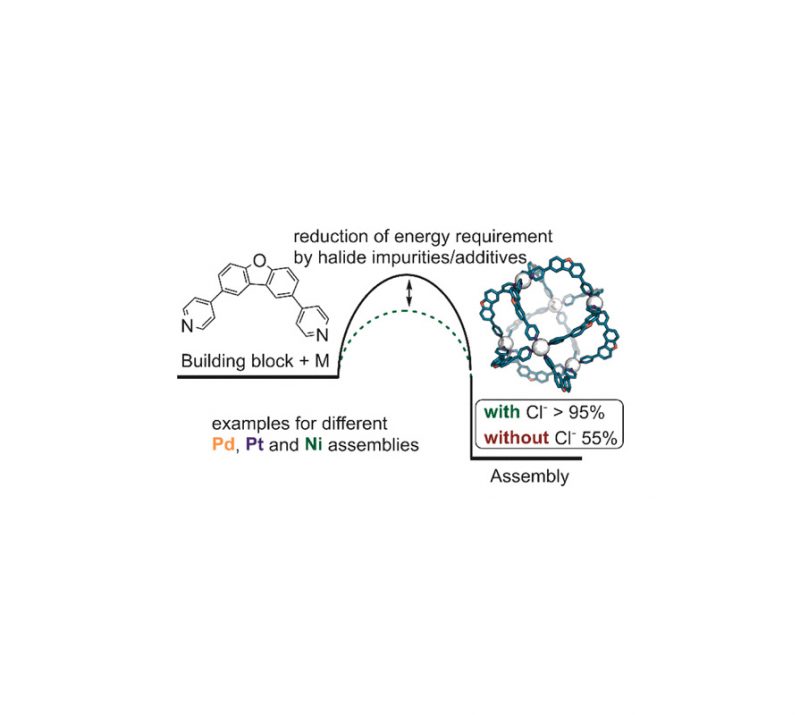
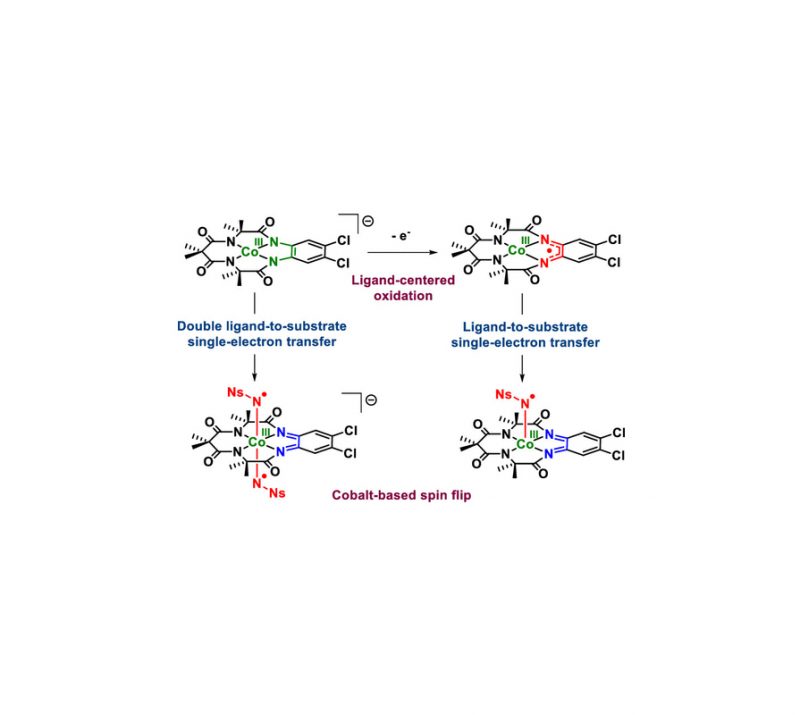
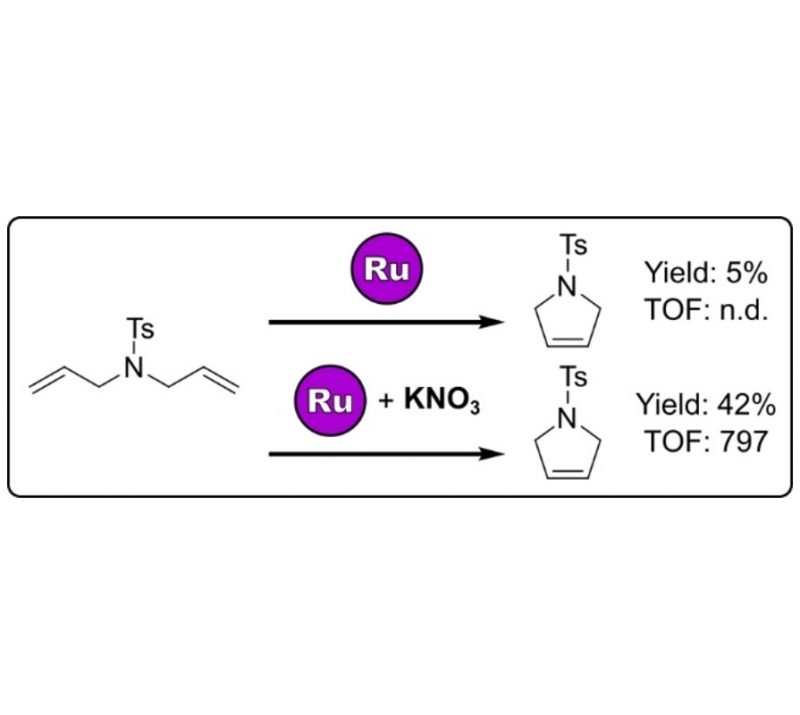
In Press
Minghui Zhou, Marianne Lankelma, Dr. Ir. Jarl Ivar van der Vlugt, Prof. Dr. Bas de Bruin
Angew. Chem. Int. Ed., 2020, 59, 2-9, accepted
DOI: 10.1002/anie.202002674
PDF: anie.202002674
Abstract
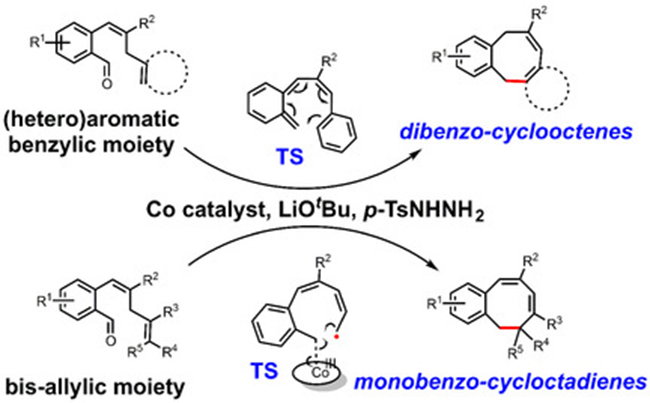
The metalloradical activation of o ‐aryl aldehydes with tosylhydrazide and a cobalt(II) porphyrin catalyst produces cobalt(III)‐carbene radical intermediates, providing a new and powerful strategy for the synthesis of medium‐sized ring structures. Herein we make use of the intrinsic radical‐type reactivity of cobalt(III)‐carbene radical intermediates in the [CoII(TPP)]‐catalyzed (TPP=tetraphenylporphyrin) synthesis of two types of 8‐membered ring compounds; novel dibenzocyclooctenes and unprecedented monobenzocyclooctadienes. The method was successfully applied to afford a variety of 8‐membered ring compounds in good yields and with excellent substituent tolerance. Density functional theory (DFT) calculations and experimental results suggest that the reactions proceed via hydrogen atom transfer from the bis‐allylic/benzallylic C−H bond to the carbene radical, followed by two divergent processes for ring‐closure to the two different types of 8‐membered ring products. While the dibenzocyclooctenes are most likely formed by dissociation of o ‐quinodimethanes (o ‐QDMs) which undergo a non‐catalyzed 8π‐cyclization, DFT calculations suggest that ring‐closure to the monobenzocyclooctadienes involves a radical‐rebound step in the coordination sphere of cobalt. The latter mechanism implies that unprecedented enantioselective ring‐closure reactions to chiral monobenzocyclooctadienes should be possible, as was confirmed for reactions mediated by a chiral cobalt‐porphyrin catalyst.