Nicolaas P. van Leest, Martijn A. Tepaske, Jean-Pierre H. Oudsen, Bas Venderbosch, Niels R. Rietdijk, Maxime A. Siegler, Moniek Tromp, Jarl Ivar van der Vlugt, and Bas de Bruin
J. Am. Chem. Soc., J. Am. Chem. Soc. 2020, 142, 1, 552-563
DOI: 10.1021/jacs.9b11715
PDF: jacs.9b11715
Abstract
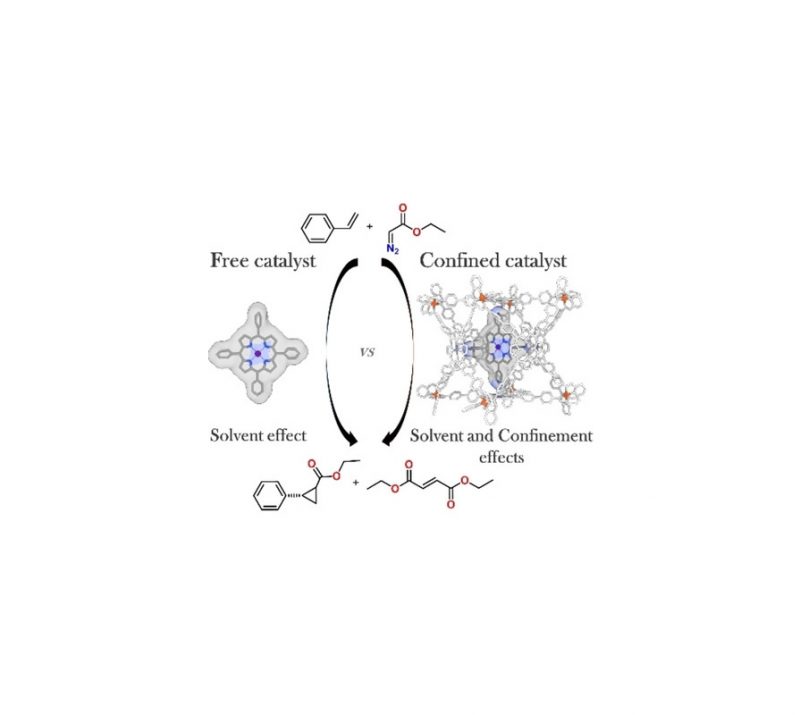
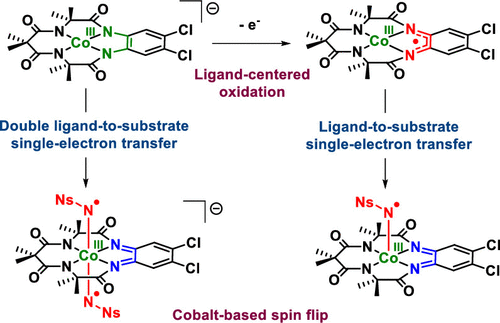
The redox noninnocence of the TAML scaffold in cobalt-TAML (tetra-amido macrocyclic ligand) complexes has been under debate since 2006. In this work, we demonstrate with a variety of spectroscopic measurements that the TAML backbone in the anionic complex [CoIII(TAMLred)]–is truly redox noninnocent and that one-electron oxidation affords [CoIII(TAMLsq)]. Multireference (CASSCF) calculations show that the electronic structure of [CoIII(TAMLsq)] is best described as an intermediate spin (S = 1) cobalt(III) center that is antiferromagnetically coupled to a ligand-centered radical, affording an overall doublet (S = 1/2) ground-state. Reaction of the cobalt(III)-TAML complexes with PhINNs as a nitrene precursor leads to TAML-centered oxidation and produces nitrene radical complexes without oxidation of the metal ion. The ligand redox state (TAMLred or TAMLsq) determines whether mono- or bis-nitrene radical complexes are formed. Reaction of [CoIII(TAMLsq)] or [CoIII(TAMLred)]– with PhINNs results in the formation of [CoIII(TAMLq)(N•Ns)] and [CoIII(TAMLq)(N•Ns)2]–, respectively. Herein, ligand-to-substrate single-electron transfer results in one-electron-reduced Fischer-type nitrene radicals (N•Ns–) that are intermediates in catalytic nitrene transfer to styrene. These nitrene radical species were characterized by EPR, XANES, and UV–vis spectroscopy, high-resolution mass spectrometry, magnetic moment measurements, and supporting CASSCF calculations.