Lukas A. Wolzak, Joen J. Hermans, Folkert de Vries, Keimpe J. van den Berg, Joost N. H. Reek, Moniek Tromp, Ties J. Korstanje
Catal. Sci. Technol., 2021, 11, 3326-3332
DOI: 10.1039/d1cy00184a
Abstract
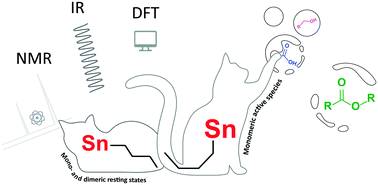
Monoalkyltin(IV) complexes are well-known catalysts for esterification reactions and polyester formation, yet the mode of operation of these Lewis acidic complexes is still unknown. Here, we report on mechanistic studies of n-butylstannoic acid in stoichiometric and catalytic reactions, analyzed by NMR, IR and MS techniques. While the chemistry of n-butyltin(IV) carboxylates is dominated by formation of multinuclear tin assemblies, we found that under catalytically relevant conditions only monomeric n-BuSn(OAc)3 and dimeric (n-BuSnOAc2OEt)2 are present. Density functional theory (DFT) calculations provide support for a mononuclear mechanism, where n-BuSn(OAc)3 and dimeric (n-BuSnOAc2OEt)2 are regarded as off-cycle species, and suggest that carbon–oxygen bond breaking is the rate-determining step.