Arnout P. T. Hartendorp, Felix J. de Zwart, Hans Bieräugel, Bas de Bruin, Joost N. H. Reek, Jan H. van Maarseveen
Organic & Biomolecular Chemistry, 2022, 20(3), 575-578
DOI: 10.1039/d1ob02309h
Abstract
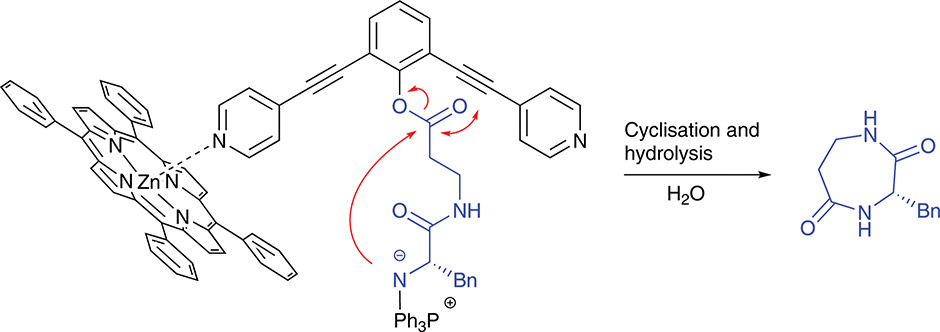
Phenol ester activated dipeptides that are reluctant to ring-close have been cyclised with the aid of sterically shielding metallo-porphyrins avoiding unwanted intermolecular reactions. The binding of ZnTPP to the dipyridine-functionalised activating phenolic ester was studied by NMR titrations and modelling. Staudinger-mediated cyclisations in the presence of ZnTPP increased the yield of the cyclic dipeptide from 16% to 40%.