Víctor Varela‐Izquierdo, Ana M. Geer, Bas de Bruin, José A. López, Miguel A. Ciriano, Cristina Tejel
Chem. Eur. J., 2019, 25, 15915-15928
DOI: 10.1002/chem.201903981
Abstract
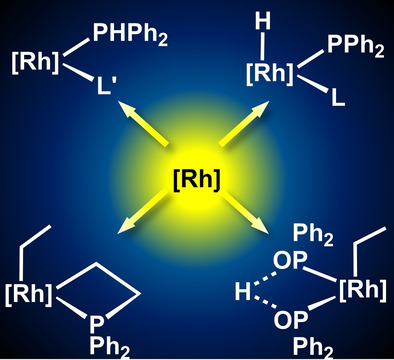
The feasibility of oxidative addition of the P−H bond of PHPh2 to a series of rhodium complexes to give mononuclear hydrido‐phosphanido complexes has been analyzed. Three main scenarios have been found depending on the nature of the L ligand added to [Rh(Tp)(C2H4)(PHPh2)] (Tp= hydridotris(pyrazolyl)borate): i) clean and quantitative reactions to terminal hydrido‐phosphanido complexes [RhTp(H)(PPh2)(L)] (L=PMe3, PMe2Ph and PHPh2), ii) equilibria between RhI and RhIII species: [RhTp(H)(PPh2)(L)]⇄[RhTp(PHPh2)(L)] (L=PMePh2, PPh3) and iii) a simple ethylene replacement to give the rhodium(I) complexes [Rh(κ2‐Tp)(L)(PHPh2)] (L=NHCs‐type ligands). The position of the P−H oxidative addition–reductive elimination equilibrium is mainly determined by sterics influencing the entropy contribution of the reaction. When ethylene was used as a ligand, the unique rhodaphosphacyclobutane complex [Rh(Tp)(η1‐Et)(κC,P‐CH2CH2PPh2)] was obtained. DFT calculations revealed that the reaction proceeds through the rate limiting oxidative addition of the P−H bond, followed by a low‐barrier sequence of reaction steps involving ethylene insertion into the Rh−H and Rh−P bonds. In addition, oxidative addition of the P−H bond in OPHPh2 to [Rh(Tp)(C2H4)(PHPh2)] gave the related hydride complex [RhTp(H)(PHPh2)(POPh2)], but ethyl complexes resulted from hydride insertion into the Rh−ethylene bond in the reaction with [Rh(Tp)(C2H4)2].