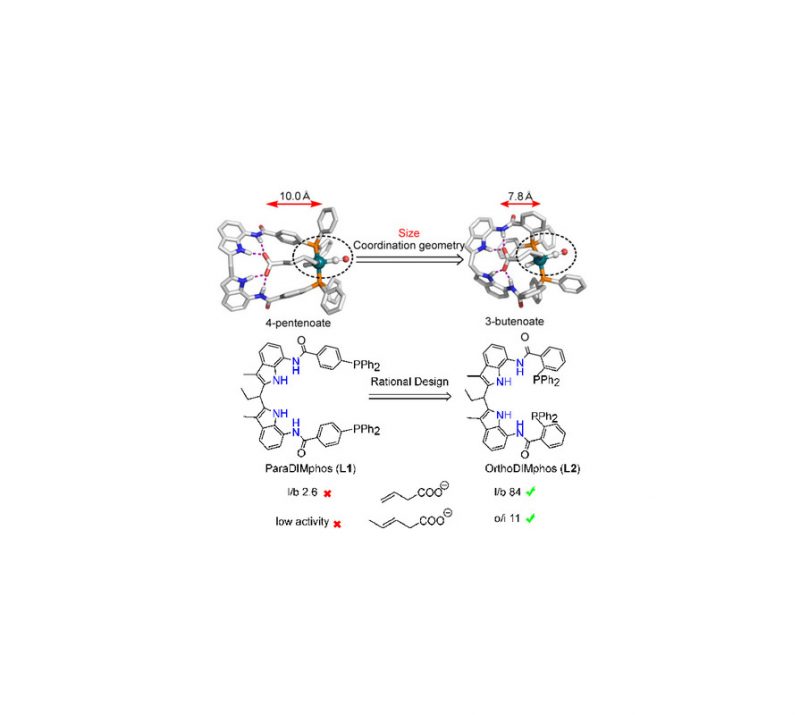
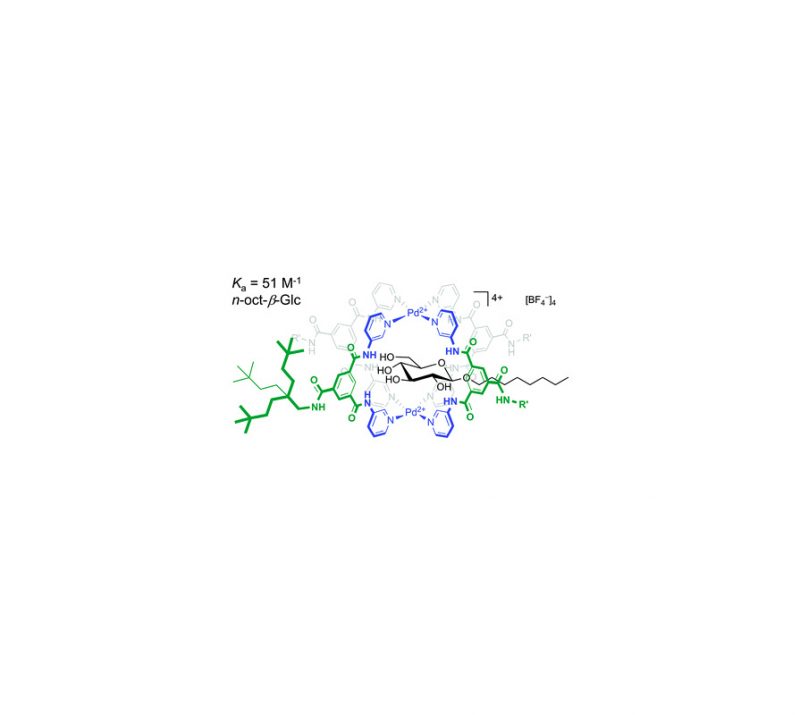
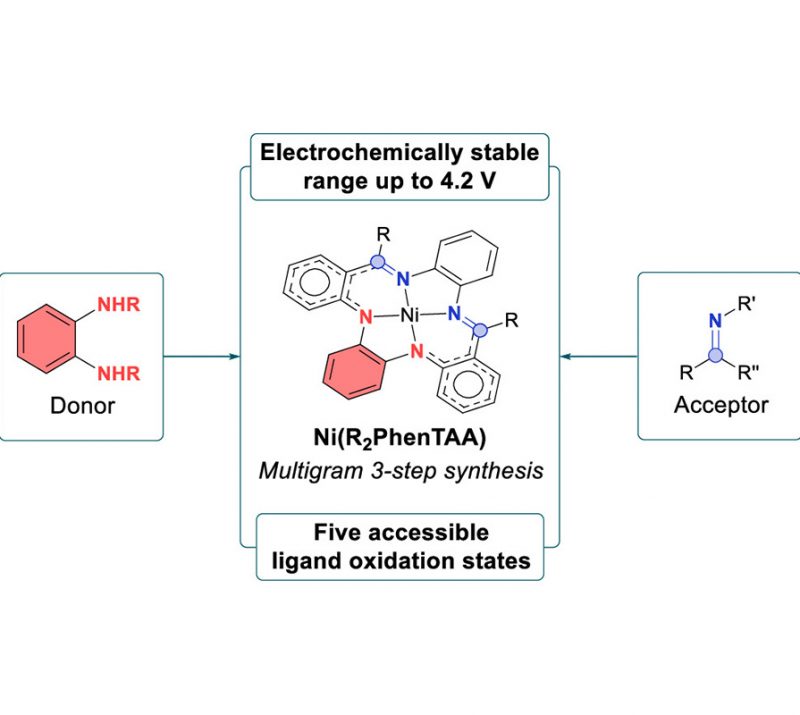
Petrus C. M. Laan, Eduard O. Bobylev, Norbert J. Geels, Gadi Rothenberg, Joost N. H. Reek, and Ning Yan
J. Phys. Chem. C, 2023, 127, 50, 24129–24136
DOI: 10.1021/acs.jpcc.3c05691
Abstract
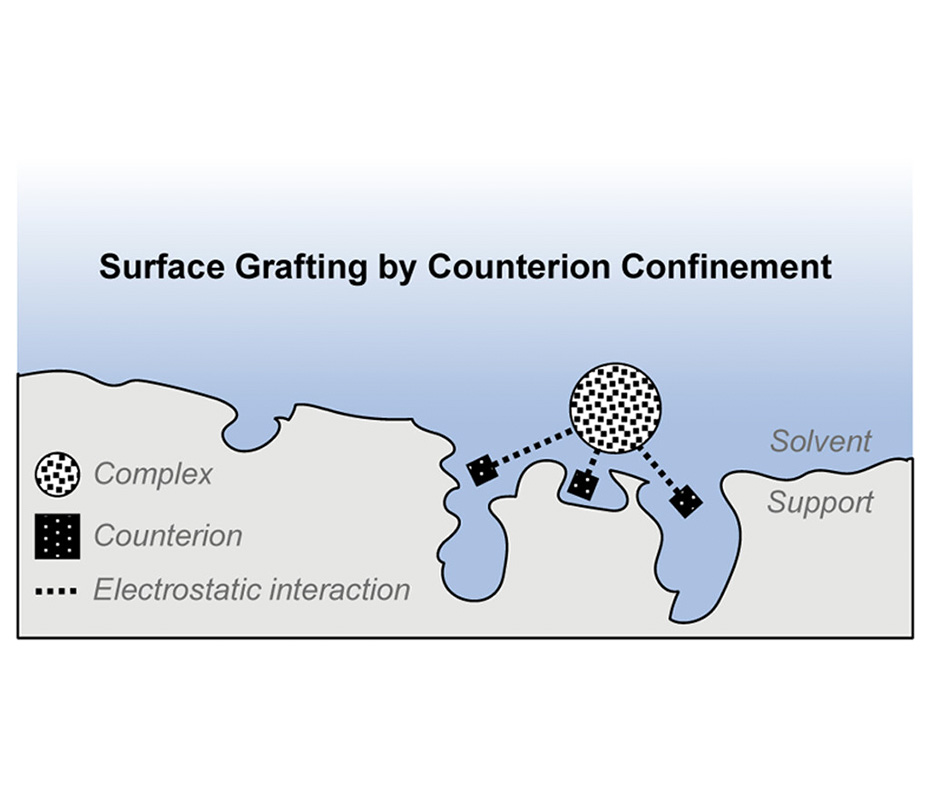
Grafting molecular complexes on solid supports is a facile strategy to synthesize advanced materials. Here, we present a general and simple method for noncovalent grafting on charge-neutral surfaces. Our method is based on the generic principle of counterion confinement in surface micropores. We demonstrate the power of this approach using a set of three platinum complexes: Pt1 (Pt1L4(BF4)2, L = p-picoline), Pt2 (Pt2L4(BF4)4, L = 2,6-bis(pyridine-3-ylethynyl)pyridine), and Pt12(Pt12L24(BF4)24, L = 4,4′-(5-methoxy-1,3-phenylene)dipyridine). These complexes share the same counterion (BF4–) but differ vastly in their size, charge, and structure. Imaging of the grafted materials by aberration-corrected high-angle annular dark-field scanning transmission electron microscopy (AC-HAADF-STEM) and energy-dispersive X-ray (EDX) showed that our method results in a homogeneous distribution of both complexes and counterions. Nitrogen sorption studies indicated a decrease in the available surface area and micropore volume, providing evidence for counterion confinement in the surface micropores. Following the adsorption of the complexes over time showed that this is a two-step process: fast surface adsorption by van der Waals forces was followed by migration over the surface and surface binding by counterion confinement. Regarding the binding of the complexes to the support, we found that the surface–adsorbate binding constant (KS) increases quadratically with the number of anions per complex up to KS = 1.6 × 106 M–1 equaling ΔG°ads = −35 kJ mol–1 for the surface binding of Pt12. Overall, our method has two important advantages: first, it is general, as you can anchor different complexes (with different charges, counterions, and/or sizes); second, it promotes the distribution of the complexes on the support surface, creating well-distributed sites that can be used in various applications across several areas of chemistry.