Shao-Tao Bai, Vivek Sinha, Alexander M. Kluwer, Pim R. Linnebank, Zohar Abiri, Bas de Bruin, Joost N. H. Reek
ChemCatChem, 2019, 11(21), 5322-5329
DOI: 10.1002/cctc.201900487
Abstract
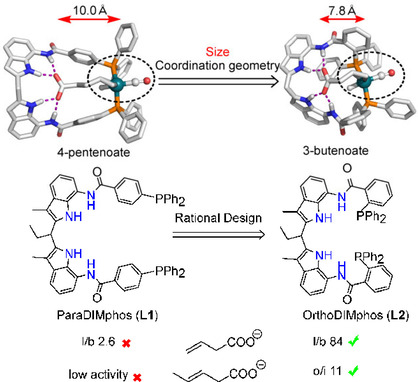
Rational design of ligands for regioselective transformations is one of the long pursuing targets in the field of transition metal catalysis. In the current contribution, we report OrthoDIMphos (L2), a ligand that was designed for regioselective hydroformylation of 3‐butenoic acid and its derivatives. The previously reported ParaDIMphos (L1) based hydroformylation catalyst was very selectively producing the linear aldehyde when substrates were bound in its pocket via hydrogen bonding. However, the distance between the binding site and the rhodium center was too large to also address 3‐butenoic acid and its derivatives. We therefore designed OrthoDIMphos (L2) as new ligand which has a shorter distance between the DIM‐receptor and the catalytic center. The OrthoDIMphos (L2) based catalyst displays high regioselectivity in the hydroformylation of 3‐butenoic acid and challenging internal alkene analogue (l/b up to 84, TON up to 630), which cannot be achieved with the ParaDIMphos (L1) catalyst. Detailed studies show that the OrthoDIMphos (L2) based catalyst forms a dimeric structure, in which the two ligands coordinate to two different rhodium metals. Substrate binding to the DIM‐receptor is required to break up the dimeric structure, and as only the monomeric analogue is a selective catalyst, the outcome of the reaction is dependent on substrate concentration used in catalysis.