Venderbosch, B.; Oudsen, J.-P. H.; Martin, D.J..; de Bruin, B.; Korstanje, T.J.; Tromp, M.
ChemCatChem, 2020, 12(3), 881-892
DOI: 10.1002/cctc.201901640
PDF: cctc.201901640
Abstract
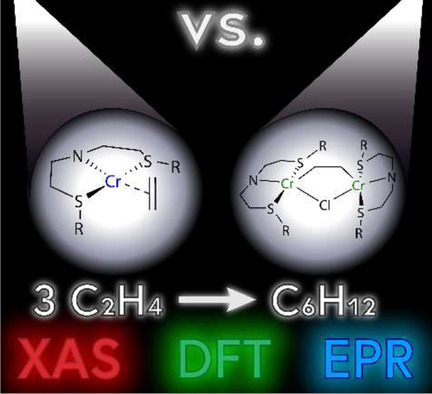
Cr‐catalyzed ethene trimerization is an industrially important process to produce 1‐hexene. Despite its industrial relevance, the changing oxidation state and the structural rearrangements of the metal center during the catalytic cycle remain unclear. In this study, we have investigated the active species in a [(R‐SN(H)S−R)CrCl3] (R=C10H21) catalyzed ethene trimerization system using a combination of spectroscopic techniques (XAS, EPR and UV/VIS) and DFT calculations. Reaction of the octahedral CrIII complex with modified methylaluminoxane (MMAO) in absence of ethene gives rise to the formation of a square‐planar CrII complex. In the presence of ethene (1 bar), no coordination was observed, which we attribute to the endergonic nature of the coordination of the first ethene molecule. Employing an alkyne as a model for ethene coordination leads to the formation of a dinuclear cationic CrIII alkyne complex. DFT calculations show that a structurally related dinuclear cationic CrIII ethene complex could form under catalytic conditions. Comparing a mechanism proceeding via mononuclear cationic CrII/CrIVintermediates to that proceeding via dinuclear cationic CrII/CrIII intermediates demonstrates that only the mechanism involving mononuclear cationic CrII/CrIVintermediates can correctly explain the observed product selectivity.