X. Yang, T. L. Gianetti, M. D. Wörle, N. P. van Leest, B. de Bruin, H. Grützmacher
Chem. Sci., 2019, 10, 1117-1125,
DOI: 10.1039/C8SC02864H
PDF: C8SC02864H
Abstract
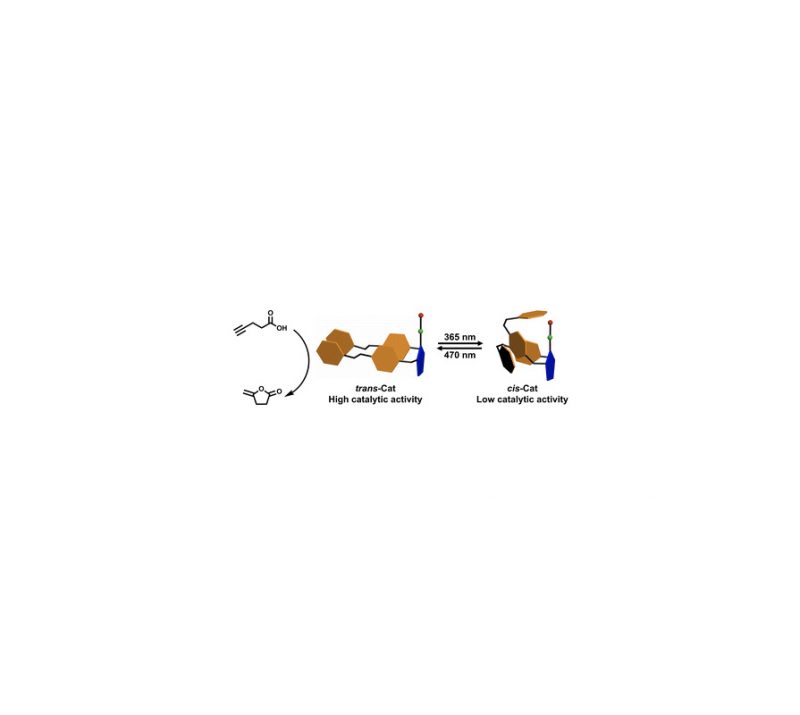
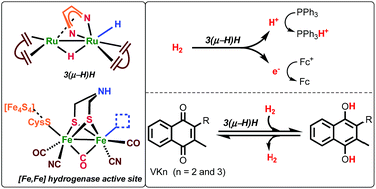
The dinuclear ruthenium complex [Ru2H(m-H)(Me2dad)(dbcot)2] contains a 1,4-dimethyl-diazabuta-1,3-diene (Me2dad) as a non-innocent bridging ligand between the metal centers to give a [Ru2(Me2dad)]core. In addition, each ruthenium is bound to one dibenzo[a,e]cyclooctatetraene (dbcot) ligand. This Rudimer converts H2to protons and electrons. It also catalyzes reversibly under mild conditions theselective hydrogenation of vitamins K2and K3to their corresponding hydroquinone equivalents withoutaffecting the C]C double bonds. Mechanistic studies suggest that the [Ru2(Me2dad)] moiety, likehydrogenases, reacts with H2and releases electrons and protons stepwise.