Petrus C. M. Laan, Felix J. de Zwart, Emma M. Wilson, Alessandro Troglia, Olivier C. M. Lugier, Norbert J. Geels, Roland Bliem, Joost N. H. Reek, Bas de Bruin, Gadi Rothenberg, and Ning Yan
ACS Catalysis, 2023, 13(13), 8467-8476
DOI: 10.1021/acscatal.3c01120
Abstract
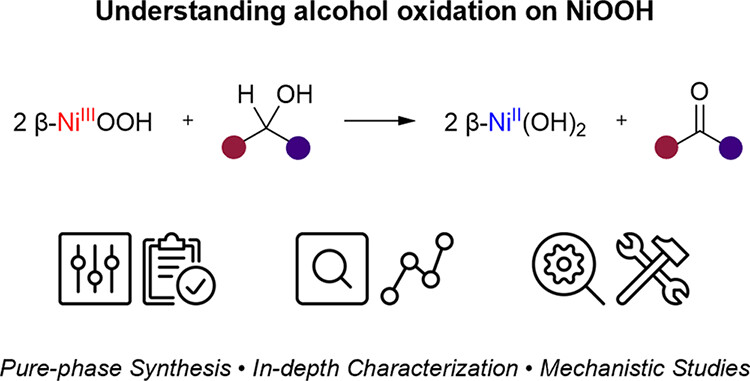
The NiOOH electrode is commonly used in electrochemical alcohol oxidations. Yet understanding the reaction mechanism is far from trivial. In many cases, the difficulty lies in the decoupling of the overlapping influence of chemical and electrochemical factors that not only govern the reaction pathway but also the crystal structure of the in situformed oxyhydroxide. Here, we use a different approach to understand this system: we start with synthesizing pure forms of the two oxyhydroxides, β-NiOOH and γ-NiOOH. Then, using the oxidative dehydrogenation of three typical alcohols as the model reactions, we examine the reactivity and selectivity of each oxyhydroxide. While solvent has a clear effect on the reaction rate of β-NiOOH, the observed selectivity was found to be unaffected and remained over 95% for the dehydrogenation of both primary and secondary alcohols to aldehydes and ketones, respectively. Yet, high concentration of OH–in aqueous solvent promoted the preferential conversion of benzyl alcohol to benzoic acid. Thus, the formation of carboxylic compounds in the electrochemical oxidation without alkaline electrolyte is more likely to follow the direct electrochemical oxidation pathway. Overoxidation of NiOOH from the β- to γ-phase will affect the selectivity but not the reactivity with a sustained >95% conversion. The mechanistic examinations comprising kinetic isotope effects, Hammett analysis, and spin trapping studies reveal that benzyl alcohol is oxidatively dehydrogenated to benzaldehyde viatwo consecutive hydrogen atom transfer steps. This work offers the unique oxidative and catalytic properties of NiOOH in alcohol oxidation reactions, shedding light on the mechanistic understanding of the electrochemical alcohol conversion using NiOOH-based electrodes.