Sergio Gonell, and Joost N. H. Reek
ChemCatChem, 2019, 11(5), 1458-1464
DOI: 10.1002/cctc.201900089
Abstract
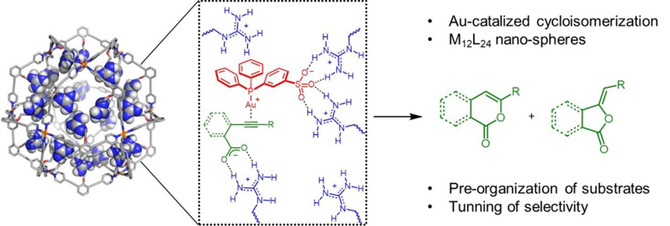
Gold‐catalyzed cycloisomerization reactions have been explored using guanidinium functionalized M12L24 nanospheres that strongly encapsulate gold complexes functionalized with a sulfonate group through hydrogen bonds. As the M12L24 nanospheres can bind up to 24 gold complexes, the effect of local catalyst concentration on the reaction outcome can be easily evaluated. Also, the guanidinium groups of the sphere can weakly interact with the carboxylic group of the substrates, facilitating the pre‐organization of the substrate near to the catalytic active site. Both effects can influence the selectivity and rate of the gold‐catalyzed transformation. Challenging acetate‐containing substrates with internal acetylene functional groups can be cyclized efficiently within the M12L24 nanospheres, where the pre‐organization of the substrate plays a crucial role. For 2‐alkynyl benzoic acids the selectivity of the reaction can be controlled by adjusting the local concentration of gold catalyst in the guanidinium functionalized M12L24 nanosphere.