

Research
- Research
- Ligand design
- Immobilized catalysts
- Supramolecular
- Collaborations
Research
The general aim of the catalysis group is the development of new catalysts for known and new important conversions, a challenge that we like to approach in a multidisciplinary fashion. Important issues that are involved in these catalytic conversions comprise of the atom-efficiency, the chemo-, regio-, and stereo-selectivity, and of course the activity and the stability of the catalyst. In addition to these aspects, we also investigate new solutions to the problem of homogeneous catalyst separation and recycling.
We have been successfully using rational ligand design and in situ spectroscopy as tools to devise new catalysts for important reactions and to unravel the mechanism of reaction pathways. We will continue this strategy for the development of new catalytic approaches. One of the factors hampering the commercialization of the newly discovered catalytic conversions is the introduction of an additional separation step required to remove the homogeneous catalyst from the product, a problem that is not an issue for heterogeneous catalysts. We have anchored catalysts to various supports, including dendrimers, hyperbranched polymers and solid supports, which has resulted in very interesting immobilized catalysts that can be separated from the product stream and reused in subsequent reactions. The interface between supramolecular chemistry and transition metal catalysis has received surprisingly little attention. It provides, however, novel and elegant strategies that could lead to new tools for the search of effective catalysts. More recently, we have introduced new supramolecular strategies in transition metal catalysis that resulted in the formation of bidentate ligands by assembly, the template assisted encapsulation of catalyst, and the efficient reversible immobilization of catalysts on dendrimers and silica support. Besides these main themes and approaches we develop new tools for combinatorial catalysis, which include both supramolecular ligand assembly and ligand synthesis on support. In addition, we are also interested in new hybrid-catalyst systems that consist of synthetic and bio-components such as enzymes and DNA.
Ligand design and in situ spectroscopy
The main objective
 of our research is the development of new catalytic processes. We try to achieve this by studying the relationship between the structure of the catalyst and its performance in catalysis. In addition to the well-known steric and electronic effects, we study the influence of the ligand geometry of the catalyst centre on the rate and selectivity of the reaction.
of our research is the development of new catalytic processes. We try to achieve this by studying the relationship between the structure of the catalyst and its performance in catalysis. In addition to the well-known steric and electronic effects, we study the influence of the ligand geometry of the catalyst centre on the rate and selectivity of the reaction.
 For example, catalytic reactions can be accelerated by forcing the geometry of the “catalyst” towards a structure that resembles the transition state, as has been proposed for metalloenzymes. Designing ligands that steer the catalytic reaction in an enantio-selective manner is a major aim of our research, although it is more based on trial-and-error and sophisticated guesses.
For example, catalytic reactions can be accelerated by forcing the geometry of the “catalyst” towards a structure that resembles the transition state, as has been proposed for metalloenzymes. Designing ligands that steer the catalytic reaction in an enantio-selective manner is a major aim of our research, although it is more based on trial-and-error and sophisticated guesses.
We have developed the Xantphos-series of wide bite angle ligands that have been used in several transition metal catalyzed reactions. In the rhodium catalyzed hydroformylation the wide bite angle results generally in very high selectivity for the linear aldehyde. Besides a high selectivity,
 POP-Xantphos gave rise to a high isomerization rate, which can be used to our advantage because internal alkenes can be converted to linear aldehydes in a cascade type reaction. The origin of the very high hydroformylation and isomerization activity of ligand POP-Xantphos became clear after measuring the rate of CO dissociation from the (diphospine)Rh(CO)2H complex using 13CO labelling in rapid-scan IR experiments. The CO dissociation rate constants k1 can be obtained by exchanging 13CO for 12CO in the (diphosphine)Rh(13CO)2H complexes. Comparison of the rate constants k1 obtained for POP-Xantphos with the ones obtained for Xantphos ligands, shows that the CO dissociation rate for ligand 33 is four to six times as high. This is a typical example of how in situ spectroscopy provides insight that can further used for ligand design.
POP-Xantphos gave rise to a high isomerization rate, which can be used to our advantage because internal alkenes can be converted to linear aldehydes in a cascade type reaction. The origin of the very high hydroformylation and isomerization activity of ligand POP-Xantphos became clear after measuring the rate of CO dissociation from the (diphospine)Rh(CO)2H complex using 13CO labelling in rapid-scan IR experiments. The CO dissociation rate constants k1 can be obtained by exchanging 13CO for 12CO in the (diphosphine)Rh(13CO)2H complexes. Comparison of the rate constants k1 obtained for POP-Xantphos with the ones obtained for Xantphos ligands, shows that the CO dissociation rate for ligand 33 is four to six times as high. This is a typical example of how in situ spectroscopy provides insight that can further used for ligand design.
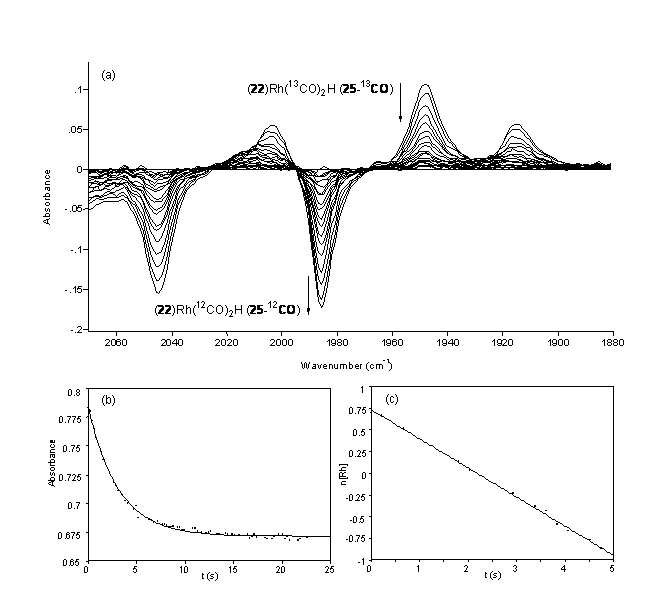
Fig. Representative difference IR spectrum (a) and kinetic data (b and c) for the 13CO dissociation from POP-Xantphos-Rh(13CO)2H (37-13CO) in the presence of unlabeled CO at 40 ºC.
People currently working on this topic
[ Franco Doro ] [ Jitte Flapper ]
Collaborations
- Prof F. Zerbetto (University of Bologna)
- Theoretical studies on asymmetric catalysis - Prof J. van Hest / Prof. A.E. Rowan (RU)Chemo-bio catalyis
- Chemo-bio catalyis
- http://www.orgchem.science.ru.nl/ - Dr. M. Bickelhaupt
- Theoretically work - Dr. Meier (UvA)
- Theoretically work
- http://www.science.uva.nl/hims/object.cfm - Prof.
Dr. C. Claver (Universitat Rovira i Virgili, Tarragona)
- Hydroformylation of alkenes; polyketone formation; asymmetric hydrogenation. - Prof. Dr. B. Chaudret (Laboratoire
de Chimie de Coordination du CNRS, lié par convention à l’Université Paul
Sabatier, Toulouse)
- Structure and reactivity of dihydrogen complexes of ruthenium and nanoparticles - Prof. Dr. D. Vogt (Eindhoven University of Technology, Schuit Institute of Catalysis, Laboratory
for Inorganic Chemistry and Catalysis)
- Hydroformylation of higher alkenes; asymmetric hydroformylation of alkenes; hydrocyanation of alkenes; hydrovinylation. - Prof.
J.G. de Vries (University of Groningen, DSM)
- Heck reaction, allylic substitution. - Prof.
dr. C.J. Elsevier (HIMS UvA):
- Olefin polymerization - Prof. Dr. B. Hessen (University of Groningen)
- Polymerization
References (PDF):
Reviews:
- P.W.N.M. van Leeuwen, P.C.J. Kamer and J.N.H. Reek The bite angle makes the catalyst. Pure Appl. Chem. 1999, 71, 1443.
- P.C.J. Kamer, J.N.H. Reek, P.W.N.M. van Leeuwen, Designing ligands with the right bite, Chemtech, 1998, September, 27.
- P.W.N.M. van Leeuwen, P.C.J. Kamer, J.N.H. Reek, and P. Dierkes Ligand bite angle effects in metal-catalyzed C-C bond formation. Chem. Rev. 2000, 100, 2741.
- P.C.J. Kamer, P.W.N.M. van Leeuwen and J.N.H. Reek, Wide, bite angle diphosphines: Xantphos ligands in transition metal complexes and catalysis Acc. Chem. Res. 2001, 34, 895.
- P.W.N.M. van Leeuwen, P.C.J. Kamer, L.A. van der Veen, and J.N.H. Reek, Bite angle effects in hydroformylation catalysis Chin. J. Chem. 2001, 19, 1.
Original contributions
- M. Schreuder Goedheijt, J.N.H. Reek, P.C.J. Kamer, P.W.N.M. van Leeuwen, A highly selective water-soluble dicationic palladium catalyst for the biphasic hydroxycarbonylation, Chem. Commun. 1998, 2431.
- R.J. van Haaren, H. Oevering, B.B. Coussens, G. P.F. van Strijdonck, J.N.H. Reek, P.C.J. Kamer, P.W.N.M. van Leeuwen, On the influence of the bite angle of bidentate phosphane ligands on the regioselectivity in allylic alkylation. Eur. J. Inorg. Chem. 1999, 1237.
- Y. Guari, D.S. van Es, J.N.H. Reek, P.C.J. Kamer and P.W.N.M. van Leeuwen, An Efficient, Palladium-Catalysed, Amination of Aryl Bromides Tetrahedron Lett. 1999 , 40, 3789.
- L.A. van der Veen, P.H. Keeven, G.C. Schoemaker, J.N.H. Reek, P.C.J. Kamer, P.W.N.M. van Leeuwen, M. Lutz, and A.L. Spek, Origin of the Bite Angle Effect in Rhodium Diphosphine Catalyzed Hydroformylation Organometallics 2000, 19, 872.
- D.G.I. Petra, J.N.H. Reek, J.-W. Handgraaf, E.J. Meijer, P. Dierkes, P.C.J. Kamer, J. Brussee, H.E. Schoemaker and P.W.N.M. van Leeuwen, Chiral induction effects in ruthenium(II)-amino alcohol catalysed asymmetric transfer hydrogenation of ketones: An experimental and theoretical approach Chem. Eur. J. 2000, 6, 2818.
- D.G.I. Petra, J.N.H. Reek, P.C.J. Kamer, H.E. Schoemaker and P.W.N.M. van Leeuwen IR-spectroscopy as a high-througput screening-technique for enantioselective hydrogen-transfer catalysts. Chem. Commun. 2000, 683.
- R.J. van Haaren, K. Druijven, G.P.F. van Strijdonck, H. Oevering, J.N.H. Reek, P.C.J. Kamer, P.W.N.M. van Leeuwen, Bite angle effect of bidentate P-N ligands in palladium catalysed allylic alkylation J. Chem. Soc. Dalton. 2000,1549-1554
- R.J. van Haaren, G.P.F. van Strijdonck, H. Oevering, J.N.H. Reek, P.C.J. Kamer, P.W.N.M. van Leeuwen, On the influence of the bite angle on the allylic alkylation of E and Z substrates: Loss and retention of double bond chemistry. Eur. J. Inorg. Chem. 2001, 3, 837.
- Y. Guari, G.P.F. van Strijdonck, M.D.K. Boele, J.N.H.Reek, P.C.J. Kamer, P.W.N.M. van Leeuwen, Palladium-Catalyzed Amination of Aryl Bromides using Bidentate Phosphorus Ligands. A Kinetic Study. Chem. Eur. J. 2001, 7, 475.
- R. J. van Haaren, K. Goubitz, J. Fraanje, G.P.F. van Strijdonck, H. Oevering, B.B. Coussens, J.N.H. Reek, P.C.J. Kamer, P.W.N.M. van Leeuwen, An X-ray study towards the steric effect of the bite angle on the geometry of palladium(allyl) complexes: implications for the regioselectivity in the allylic alkylation. Inorg. Chem. 2001, 40, 3363.
- A. Hage, D.G.I. Petra, J.A. Field, D. Schipper, J.B.P.A. Wijnberg, P.C.J. Kamer, J.N.H. Reek, P.W.N.M. van Leeuwen, R. Wever, H.E. Schoemaker, Asymmetric reduction of ketones via whole cell bioconversions and transfer hydrogenation: complementary approaches. Tetrahedron: Asymmetry 2001, 12, 1025-1034.
- C.B. Dieleman, J.N.H. Reek, P.C.J. Kamer, P.W.N.M. van Leeuwen, Xantphite: a new family of ligands for catalysis Helv. Chim. Acta 2001, 84, 3269.
- R.J. van Haaren, P.H. Keeven, L.A. van der Veen, K. Goubitz, G.P.F. van Strijdonck, H. Oevering, J.N.H. Reek, P.C.J. Kamer, P.W.N.M. van Leeuwen, The effect of ligand donor atoms on the regioselectivity in the palladium catalyzed allylic alkylation Inorg. Chim. Acta 2002, 327, 108.
- R.J. van Haaren, J.N.H. Reek, H. Oevering, B.B. Coussens, G.P.F. van Strijdonck, P.C.J. Kamer, P.W.N.M. van Leeuwen, Teaching organometallic chemistry using computational chemistry J. Chem. Educ. 2002 588-591.
- M.A. Zuideveld, B.H.G. Swennenhuis, M.D.K. Boele, Y. Guari, G.P.F. van Strijdonck, J.N.H. Reek, P.C.J. Kamer, K. Goubitz, J. Fraanje, M. Lutz, A.L. Spek, P.W.N.M. van Leeuwen, J. Chem. Soc. Dalton. 2002, 2308.
- F. Zoraida, M.S. Beentjes, G.D. Batema, C.B. Dieleman, G.P.F. van Strijdonck, J.N.H. Reek, P.C.J. Kamer, J. Fraanje, K. Goubitz, P.W.N.M. van Leeuwen, SPANphos: A C2-symmetric trans-coordinating diphosphane ligand. Angew. Chem. Int. Ed. 2003 42, 1284-1287.
- J-W. Handgraaf, J.N.H. Reek, E.-J. Meijer, Iridium(I) versus Ruthenium(II). A Computational Study of the Transition Metal Catalyzed Transfer Hydrogenation of Ketones. Organometallics 2003 22 3150.
- V.F. Slagt, P.W.N.M. van Leeuwen, J.N.H. Reek*, Multicomponent porphyrin assemblies as functional bidentate phosphite ligands for regioselective rhodium-catalyzed hydroformylation. Angew. Chem. Int. Ed. 2003, 42, 5619.
- V.F. Slagt, P.W.N.M. van Leeuwen, J.N.H. Reek* Bidentate ligands formed by self-assembly, Chem. Commun. 2003, 2474.
- V. F. Slagt, P. C. J. Kamer, P. W. N. M. van Leeuwen, J. N. H. Reek* Encapsulation of Transition Metal Catalysts by Ligand-Template Directed Assembly, J. Am. Chem. Soc. 2004, 126, 1526.
- V. F. Slagt, M. Röder, P. C. J. Kamer, P. W. N. M. van Leeuwen, J. N. H. Reek* Supraphos: A Supramolecular Strategy To Prepare Bidentate Ligands. J. Am. Chem. Soc. (communication) 2004, 126, 4056.
Immobilized catalysts
Besides the development of new catalysts by rational ligand design and combinatorial approaches, a lot of research is devoted to the complete system approach including catalyst recycling. Various elegant concepts for catalyst separation and recycling have been developed by us and others. Two phase-catalysis is a very general method and involves the immobilization of the catalyst in a solvent that is immiscible with the product phase. The introduction of water-soluble groups to the ligands of a transition metal allow the catalyst to be immobilized in an aqueous phase. A widely studied approach to facilitate catalyst-product separation is the attachment of homogeneous catalysts to dendritic, polymeric organic, inorganic or hybrid supports. Here the ligand is functionalized with a group that enables anchoring to such a support.
Unfortunately, there is no “holy grail” that provides a general solution for catalyst-separation, and for every new catalytic process the strategy for catalyst separation and recycling needs to be studied. General properties, such as solubility of the substrates, catalyst instability and metal leaching during the recycling procedure strongly limit some of the concepts. For example, aqueous-phase catalysis is limited to substrates that are soluble in water. Immobilization to inorganic materials such as silica has advantages due to physical strength and chemical inertness, but the activity is generally lower than the homogeneous system. The decrease in activity is usually not a problem for catalysts on soluble dendrimeric or hyperbranched polymer supports, but here the availability of suitable membrane materials complicates practical use. We have been exploring two-phase catalysis, dendrimer supported catalysis and catalysis using silica immobilized catalysts.
Dendritic transition metal catalysis
Dendrimers are appealing molecules with well-defined structures in the nanometer dimension. We prepared
both core- and periphery-functionalized dendritic catalysts that were applied as catalyst for several reactions in continuous-flow membrane reactors. The specific micro-environment around the catalyst in core-functionalized systems resulted in enzyme-like catalyst properties: substrate size-selectivity and a different product distribution were observed, and in addition it was found that the catalyst was stabilized due to the site-isolation. (Chem Commun 1999, Chem Commun 1999, Angew. Chem. 2001, Chem Rev. 2002 Rev. Mol. Biotech., 2002, J. Am. Chem. Soc. 2004)
Fig. Two dendritic catalyst in which the catalyst is located at the core (Top) and at the periphery (Bottom) of the dendrimer.
Immobilization of catalysts on silica support
Rhodium-diphosphine complexes were immobilized on a silica support via several approaches including Supported Aqueous Phase Catalyst (Chem Commun. 1999), and also via covalent anchoring in a sol-gel matrix or to the surface of commercially available silica (Angew. Chem. 1999). In the hydroformylation of 1-octene these systems showed a high selectivity for the linear aldehyde and the catalyst activity and selectivity remained unaltered upon multiple recycling. The catalyst-support interactions were controlled by manipulation of the reaction conditions directing the catalyst function; the catalyst was switched between hydroformylation, hydrogenation and hydroformylation-hydrogenation cascade reactions (JACS 2001, Chem. Eur. J. 2001).

People currently working on this topic
-
Collaborations
- Prof. Poliakoff (University of Nottingham)
- Catalysis in supercritical CO2 - Prof. Dr. D. Vogt (Eindhoven University of Technology, Schuit Institute of Catalysis, Laboratory
for Inorganic Chemistry and Catalysis)
- Hydroformylation of higher alkenes; asymmetric hydroformylation of alkenes; hydrocyanation of alkenes; hydrovinylation. - Prof. G. van Koten (University of Utrecht)
- Carbosilane dendrimers as support for homogeneous catalysts. - Prof.
Dr. de Haan (Technical University of Twente)
- New separation methods and reactor design for homogeneous catalysis.
References (PDF):
Reviews:
- M. J. Wilkinson, P. W. N. M. van Leeuwen, J. N. H. Reek* Perspective article “New directions in supramolecular transition metal catalysis” Org. Bio. Chem. 2005 3, 2371.
- J.N.H. Reek*, D. de Groot, G.E. Oosterom, P.C.J. Kamer, P.W.N.M. van Leeuwen, Core and periphery functionalized dendrimers for transition metal catalysis; a covalent and a non-covalent approach Rev. Mol. Biotech. 2002, 90, 159.
- G.E. Oosterom, J.N.H. Reek,* P.C.J. Kamer, and P.W.N.M. van Leeuwen,* Transition metal catalysis using functionalized dendrimers. Angew. Chem. Int. Ed. Engl. 2001, 40, 1828.
- P.W.N.M. van Leeuwen, A.J. Sandee, J.N.H. Reek and P.C.J. Kamer, Xantphos-based, silica-supported, selective, and recyclable hydroformylation catalysts: a review, Journal of Molecular Catalysis A. 2002, 182-183, 107.
- R. van Heerbeek, P.C.J. Kamer, P.W.N.M. van Leeuwen, J. N. H. Reek,* Chem. Rev., 2002, 102,3717.
Original contributions
- A.J. Sandee, V.C. Slagt, J.N.H. Reek, P.C.J. Kamer and P.W.N.M. van Leeuwen A stable and recyclable supported aqueous phase catalyst for highly selective hydroformylation of higher olefins Chem. Commun. 1999, 1633.
- G.E. Oosterom, R.J. van Haaren, J.N.H. Reek, P.C.J. Kamer and P.W.N.M. vanLeeuwen Catalysis in the core of a carbosilane dendrimer. Chem. Commun. 1999, 1119.
- A.J. Sandee, L.A. van der Veen, J.N.H. Reek, P.C.J. Kamer, M. Lutz, A.L. Spek and P.W.N.M. van Leeuwen A robust, environmentally benign catalyst for highly selective hydroformylation. Angew. Chem. Int. Ed. Engl. 1999, 38, 3231.
- D. de Groot, E.B. Eggeling, J.C. de Wilde, H. Kooijman, R.J. van Haaren, A.W. van der Made, A.L. Spek, D. Vogt, J.N.H. Reek, P.C.J. Kamer and P.W.N.M. van Leeuwen Palladium complexes of phosphine functionalised carbosilane dendrimers as catalysts in a continuous flow membrane reactor Chem. Commun. 1999, 1623.
- R. van Heerbeek, J.N.H. Reek, P.C.J. Kamer and P.W.N.M. van Leeuwen, Divergent synthesis of carbosilane wedges as dendritic building blocks: a new strategy towards core functionalised carbosilane dendrimers Tetrahedron Lett 1999, 40, 7127.
- M. Schreuder Goedheijt, B.E. Hanson, J.N.H. Reek, P.C.J. Kamer, P.W.N.M. van Leeuwen, Spontaneous Formation of Vesicles from Amphiphilic Diphosphines: A Highly Selective and Recyclable Rhodium Catalyst. J. Am. Chem. Soc. 2000, 122, 1650.
- P.W.N.M van Leeuwen, P.C.J. Kamer and J.N.H. Reek, Water, the panacea in homogeneous catalysis. CATTECH 2000, 6, 164.
- N.J. Meehan, A.J. Sandee, J.N.H Reek, P.C.J. Kamer, P.W.N.M. van Leeuwen, M. Poliakoff, Continuous, selective hydroformylation in supercritical carbon dioxide using an immobilised homogeneous catalyst Chem. Commun. 2000, 1497-1498.
- D. de Groot, P. G. Emmerink, C. Coucke, J.N.H. Reek,* P.C.J. Kamer, and P.W.N.M. van Leeuwen,* Rhodium catalysed hydroformylation using diphenylphosphine functionalised carbosilane dendrimers, Inorg. Chem. Commun., 2000, 3, 711.
- A.J. Sandee, D.G.I. Petra, J.N.H. Reek,* P.C.J. Kamer, and P.W.N.M. van Leeuwen* Solid phase synthesis of homogeneous ruthenium catalysts on silica for the continuous asymmetric transfer hydrogenation reaction Chem. Eur. J. 2001, 7, 1202.
- A.J. Sandee, R.S. Ubale, M. Makkee, J.N.H. Reek, P.C.J. Kamer, J.A. Moulijn, P. W.N.M. van Leeuwen ROTACAT: a rotating device containing a designed catalyst for highly selective hydroformylation. Adv. Synt. Catal. 2001, 1, 201.
- J.N.H. Reek, A.J. Sandee, M. Schreuder Goedheijt, P.C.J. Kamer, P.W.N.M Van Leeuwen, Recyclable hydroformylation catalysts of higher alkenes using immobilized catalysts and two-phase systems. Erdoel, Erdgas, Kohle 2001, 117, 134-138.
- D. de Groot, B.F.M de Waal, J.N.H Reek,* A.P.H.J. Schenning, P.C.J. Kamer, E.W. Meijer,* P.W.N.M. van Leeuwen, Noncovalently Functionalized Dendrimers as Recyclable Catalysts. J. Am. Chem. Soc. 2001, 123, 8453.
- A.J. Sandee, J.N.H. Reek,* P.C.J. Kamer, and P.W.N.M. van Leeuwen*, A Silica-Supported, Switchable, and Recyclable Hydroformylation-Hydrogenation Catalyst J. Am. Chem. Soc. 2001, 123, 8468.
- G.E. Oosterom, S. Steffens, J.N.H. Reek*, P.C.J. Kamer, P.W.N.M. van Leeuwen*, Core-functionalized Dendrimeric Mono- and Diphosphine Rhodium Complexes; Application in Hydroformylation and Hydrogenation, Topics in Catalysis, 2002, 19,61.
- D. de Groot, J.N.H. Reek*, P.C.J. Kamer, P.W.N.M. van Leeuwen*, Palladium Complexes of Phosphane-Functionalised Carbosilane Dendrimers as Catalysts in a Continuous-Flow Membrane Reactor Eur. J. Org. Chem. 2002, 1085.
- W.P. Mul, K. Ramkisoensing, P.C.J. Kamer, J.N.H. Reek, A.J. van der Linden, A. Marson, and P.W.N. M. van Leeuwen, New, highly efficient work-up protocol for sulfonated diphosphines. Adv. Synth. Cat. 2002, 344, 293.
- A.J. Sandee, D. Dimitrijevic, R.J. van Haaren, J.N.H. Reek*, P.C.J. Kamer, P.W.N.M. van Leeuwen*, Silica immobilised palladium phosphine complexes as recyclable, regioselective catalysts for the allylic alkylation, Journal of Molecular Catalysis A. 2002, 182-183, 309.
- J.N.H. Reek*, D. de Groot, G.E. Oosterom, P.C.J. Kamer, P.W.N.M. van Leeuwen, Phosphine-functionalized dendrimers for transition-metal catalysis. Comptes Rendus Chimie 2003, 6, 1061.
- P. N. M. Botman, A. Amore, R. van Heerbeek, J. W. Back, H. Hiemstra, J.N.H. Reek,* J. van Maarseveen,* Dendritic phosphoramidite ligands in Rh-catalysed asymmetric hydrogenations, Tet. Lett. 2004, 45, 5999.
- R. P. J. Bronger, J. P. Bermon, J. N. H. Reek, P. C. J. Kamer, P. W. N. M. van Leeuwen, D. N. Carter, P. Licence, M. Poliakoff, The immobilisation of phenoxaphosphine-modified xanthene-type ligand on polysiloxane support and application thereof in the hydroformylation reaction, J. Mol. Cat. A: 2004, 224, 145-152.
- R. Chen, R.P.J. Bronger, P. C. J. Kamer, P. W. N. M. van Leeuwen, J. N. H. Reek* “Noncovalent anchoring of Homogeneous Catalysts to Silica Supports with well-defined Binding Sites” J. Am. Chem. Soc. 2004, 126, 14557.
- C. Muller, L. J. Ackerman, J. N. H. Reek,* P. C. J. Kamer, P. W. N. M. van Leeuwen* “Site-Isolation Effects in a Dendritic Nickel Catalyst for the Oligomerization of Ethylene“ J. Am. Chem. Soc. 2004, 126, 14960.
- L. Leclercq, F. Hapiot, S. Tilloy, K. Ramkisoensing, J. N. H. Reek, P.W.N.M. van Leeuwen E. Monflier, “Sulfonated Xantphos ligand and Methylated Cyclodextrin: A winning combination for rhodium-catalyzed hydroformylation of higher olefins in aqueous medium”, Organometallics 2005 24 2070.
Supramolecular strategies in transition metal catalysis
In a recent contribution we reported the template assisted encapsulation of transition metal catalysts in hemi-spherical assemblies (Figure 1, Angew. Chem. 2001). 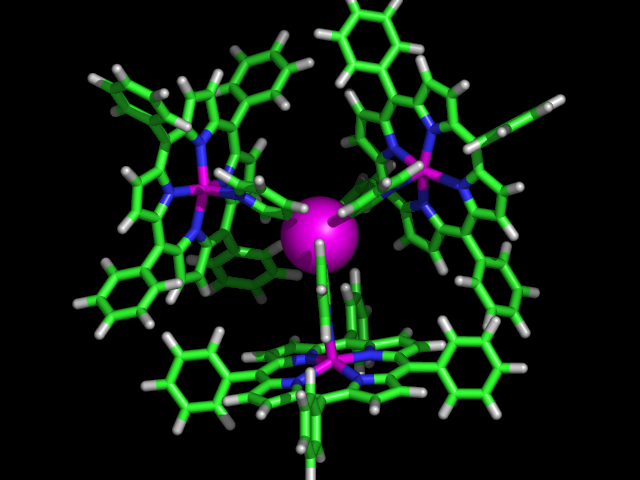 Interestingly, the catalytic performance of the encapsulated transition metal complex was significantly different from the non-encapsulated analogue, changing key features as selectivity and activity, indicating that this is a versatile supramolecular approach to new catalyst systems. In the hydroformylation of 1-octene the capsule gave an unprecedented high selectivity for the branched aldehyde, which could partly be attributed to the encapsulation of the catalyst (J. Am. Chem. Soc. 2004). A more complicated multicomponent assembly based on two trisporphyrin phosphites and three dabco-molecules was also studied and shown to give a high selectivity for the linear aldehyde (Angew. Chem. Int. Ed. 2003).
Interestingly, the catalytic performance of the encapsulated transition metal complex was significantly different from the non-encapsulated analogue, changing key features as selectivity and activity, indicating that this is a versatile supramolecular approach to new catalyst systems. In the hydroformylation of 1-octene the capsule gave an unprecedented high selectivity for the branched aldehyde, which could partly be attributed to the encapsulation of the catalyst (J. Am. Chem. Soc. 2004). A more complicated multicomponent assembly based on two trisporphyrin phosphites and three dabco-molecules was also studied and shown to give a high selectivity for the linear aldehyde (Angew. Chem. Int. Ed. 2003).
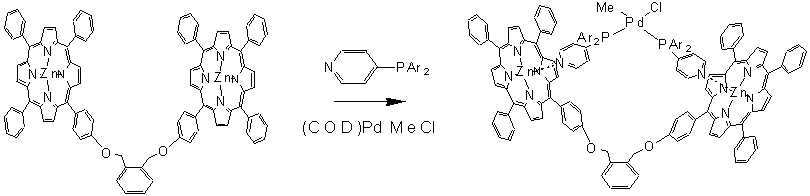
Fig.1: A computer generated picture and video (to open click image) of metal-encapsulated porphyrins and an in situ assembly of a bidentate ligand using metal-ligand interactions (right).
After these initial results we studied the formation of bidentate ligands by assembly. A three-component system based on a bisporphyrin and pyridine functionalized phosphines (and phosphates) form chelating bidentate ligands that give increased (enantio-) selectivity in rhodium catalyzed hydroformylation (Figure 1, Chem. Commun. 2003). Two component assemblies based on phosphite functionalized porphyrins pyridine functionalized phosphorus ligand clearly show the advantage of the supramolecular approach to form bidentate ligands. We introduced the term supraphos for this class of ligands since bidentate ligands form by self-assembly (figure 2).
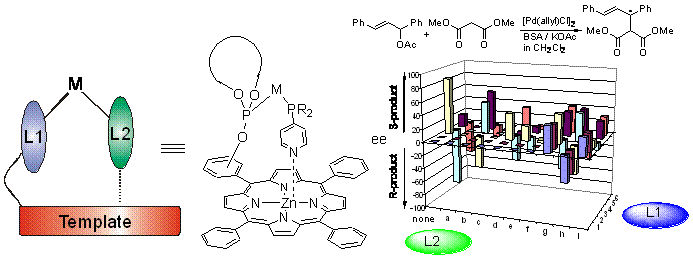
Fig.2: Schematic presentation of supraphos, the formation of a bidentate by assembly, and the catalytic results in the allylic substitution of the small supraphos library.
The power of the concept was demonstrated by the ligand library of 60, formed by simply mixing two components from the 15 building blocks synthesized (J. Am. Chem. Soc. Communication 2004, patent application, 2003). The library was used in three different reactions demonstrating the versatility of the concept. More recently, within the VICI-program, we have extended these approaches to zinc(II)salphen building blocks which show a higher affinity for nitrogen donors. We also found that these supramolecular complexes can be crystallized relatively easily, facilitating detailed structural characterization. As typical examples three of these structures are shown in figure 3, of which we are currently exploring the behaviour in catalysis.
In a different approach we have prepared and studied amphiphilic catalysts that spontaneously form aggregates in aqueous solution. These organized structures create apolar environments within the aqueous phase, accelerating reactions with hydrophobic substrates (J. Am. Chem. Soc. 2000).
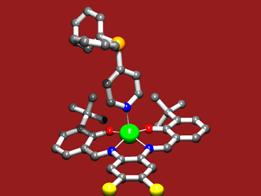
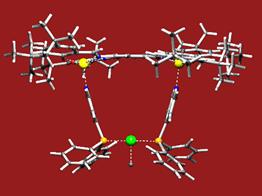
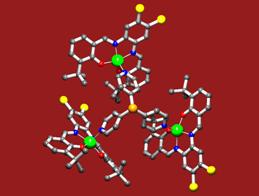
Fig.3: The X-ray structures of various supramolecular complexes in which (bis) zinc(II)salphen building blocks function as a template to associate a pyridyldiphenylphosphine (top left), a trans-palladium complex thereof (middle) and encapsulate a tris-(pyridyl)phosphine (bottom right).
We also reported the first example of efficient reversible functionalization of the periphery of a dendrimer with catalytic sites using non-covalent interactions. The supramolecular complex with 32 catalytic sites was efficiently recycled by performing the palladium catalyzed allylic amination reaction in a continuous-flow membrane reactor. (J. Am. Chem. Soc. 2001). This concept is further extended to silica type materials (J. Am. Chem. Soc. 2004), which is a topic under current investigation in combination with reactor design (A collaboration with Prof de Haan, University of Twente).
People currently working on this topic
[ Frederic Patureau ] [ Pierre Alain Breuil ] [ Jurjen Meeuwissen] [ Vladica Bocokic ] [ Johanneke Ernsting ]
Collaborations
- Prof Sanders (Cambridge)
- Supramolecular porphyrin chemistry - Prof Berkessel (Universität zu Köln)
- Chiral porphyrins - Prof J. Kikuchi Nara (Institute of science and technology Japan)
- Catalytically active Cerasomes
- http://www.jaist.ac.jp/ - Prof. Dr. E.W. Meier, Dr. A.P.H.J. Schenning (TUE)
- Development of novel catalysts for the preparation of new photoactive polymeric materials. - Prof J. van Hest / Prof. A.E. Rowan (RU)
- Chemo-bio catalyis
- http://www.orgchem.science.ru.nl/ - Dr. R.P. Sijbesma (TUE)
- Sonor catalysis, Supramolecular catalyst immobilization
- http://www.chem.tue.nl/smo/default.htm - Prof L. de Cola (UvA)
- Dendrimers and supramolecular chemistry
- http://www.science.uva.nl/hims/object.cfm - Prof.
Dr. R.J.M. Nolte / Prof A.E. Rowan (University of Nijmegen)
- Supramolecular catalysis.
- http://www.orgchem.science.ru.nl
References (PDF):
Reviews:
- M. J. Wilkinson, P. W. N. M. van Leeuwen, J. N. H. Reek* Perspective article “New directions in supramolecular transition metal catalysis” Org. Bio. Chem. 2005 DOI: 10.1039/(b503407h).
- J.N.H. Reek*, D. de Groot, G.E. Oosterom, P.C.J. Kamer, P.W.N.M. van Leeuwen, Core and periphery functionalized dendrimers for transition metal catalysis; a covalent and a non-covalent approach Rev. Mol. Biotech. 2002, 90, 159.
- R. van Heerbeek, P.C.J. Kamer, P.W.N.M. van Leeuwen, J. N. H. Reek,* Chem. Rev., 2002, 102, 3717
Patents:
- J.N.H. Reek, R.F. Chen, P.C.J. Kamer, V.C. Slagt, P.W.N.M. van Leeuwen, Patent application “Coordination complex system comprising building blocks“ 03076827.0-2104 (2003)
Original contributions
- R. van Heerbeek, J.N.H. Reek, P.C.J. Kamer and P.W.N.M. van Leeuwen, Divergent synthesis of carbosilane wedges as dendritic building blocks: a new strategy towards core functionalised carbosilane dendrimers Tetrahedron Lett 1999, 40, 7127.
- M. Schreuder Goedheijt, B.E. Hanson, J.N.H. Reek, P.C.J. Kamer, P.W.N.M. van Leeuwen, Spontaneous Formation of Vesicles from Amphiphilic Diphosphines: A Highly Selective and Recyclable Rhodium Catalyst. J. Am. Chem. Soc. 2000, 122, 1650.
- D. de Groot, B.F.M de Waal, J.N.H Reek,* A.P.H.J. Schenning, P.C.J. Kamer, E.W. Meijer,* P.W.N.M. van Leeuwen, Noncovalently Functionalized Dendrimers as Recyclable Catalysts. J. Am. Chem. Soc. 2001, 123, 8453.
- V.C. Slagt, J.N.H. Reek*, P.C.J. Kamer and P.W.N.M. van Leeuwen, Assembly of Encapsulated Transition Metal Catalysts, Angew. Chem. Int. Ed., 2001, 40, 4271-4274 (Front cover)
- J.J.L.M. Cornelissen, R. van Heerbeek, P.C. J. Kamer, J.N.H. Reek, N.A.J.M. Sommerdijk, and R.J.M. Nolte, Silver Nanoarrays templated by Blockcopolymers of Carbosilane-Dendrimers and Polyisocyanopeptides, Adv. Mater., 2002, 14, 489.
- V.F. Slagt, P.W.N.M. van Leeuwen, J.N.H. Reek* Bidentate ligands formed by self-assembly, Chem. Commun. 2003, 2474.
- V.F. Slagt, P.W.N.M. van Leeuwen, J.N.H. Reek*, Multicomponent porphyrin assemblies as functional bidentate phosphite ligands for regioselective rhodium-catalyzed hydroformylation. Angew. Chem. Int. Ed. 2003, 42, 5619.
- V. F. Slagt, P. C. J. Kamer, P. W. N. M. van Leeuwen, J. N. H. Reek* Encapsulation of Transition Metal Catalysts by Ligand-Template Directed Assembly, J. Am. Chem. Soc. 2004, 126, 1526
- V. F. Slagt, M. Röder, P. C. J. Kamer, P. W. N. M. van Leeuwen, J. N. H. Reek* Supraphos: A Supramolecular Strategy To Prepare Bidentate Ligands. J. Am. Chem. Soc. (communication) 2004, 126, 4056.
- A. Dirksen, U. Hahn, F. Schwanke, J. N. H. Reek, F. Vögtle, L. De Cola, Multiple Recognition of Barbiturate Guests by “Hamilton” Receptor-Functionalized Dendrimers, Chem. Eur. J. 2004, 10, 2036.
- J. J. L. M Cornelissen,. M. Fischer, R. van Waes, R. van Heerbeek, P. C. J.; Kamer, J. N. H. Reek, N. A. J. M. Sommerdijk, R.J.M. Nolte, Synthesis, characterization and aggregation behavior of block copolymers containing a polyisocyanopeptide segment, Polymer, 2004, 45, 7417-7430.
- R. Chen, R.P.J. Bronger, P. C. J. Kamer, P. W. N. M. van Leeuwen, J. N. H. Reek* “Noncovalent anchoring of Homogeneous Catalysts to Silica Supports with well-defined Binding Sites” J. Am. Chem. Soc. 2004, 126, 14557.
- A. W. Kleij, M. Kuil, D.M. Tooke, M. Lutz, A. L. Spek, J. N.H. Reek * “Zn-Salphen Complexes as Versatile Building Blocks for the Construction of Supramolecular Box Assemblies” Chem. Eur. J., 2005, DOI 10.1002/chem.200500227
- J.N.H. Reek *, M. Röder, P. E. Goudriaan, P. C.J. Kamer, P. W.N.M. van Leeuwen, V. F. Slagt, “Supraphos: a supramolecular strategy to prepare bidentate ligands” J. Organometal. Chem. 2005, DOI: 10.1016/j.jorganchem.2005.02.026
- A. W. Kleij, M. Lutz, A. L. Spek, P.W. N. M. van Leeuwen, J. N. H. Reek* “Encapsulated Transition Metal Catalysts Comprising Peripheral Zn(II)salen Building Blocks: Template-Controlled Reactivity and Selectivity in Hydroformylation Catalysis” Chem. Commun. 2005 (Advance Article) DOI: 10.1039/b503708e
- Xiao-Bin Jiang, Laurent Lefort, P. Elsbeth Goudriaan, André H. M. de Vries, Piet W. N. M. van Leeuwen, Johannes G. de Vries, Joost N. H. Reek* “Screening of a Supramolecular Catalyst Library in the Search for Selective Catalysts for the Asymmetric Hydrogenation of a Difficult Enamide Substrate” Angew. Chem. Int. 2006. Published online, DOI: 10.1002/anie.200503663
Collaborations
International Collaborations
- Prof. Hansjörg Grutzmacher, ETH Zürich (Switzerland)
- Prof. Peter Budzelaar, University of Manitoba (Canada)
- Dr. Ed Reijerse, MPI für Bioanorganische Chemie, Mülheim a/d Ruhr (Germany)
- Prof. Kin-Shing Chan, Chinese University of Hong Kong (China)
- Prof. Miguel Ciriano & Dr. Cristina Tejel, University of Zaragoza (Spain)
- Prof. Sven Schneider, University of Göttingen (Germany)
- Dr. Robert Wolf, University of Regensburg (Germany)
- Prof. X. Peter Zhang, University of South California (USA)
- Prof. Xuefeng Fu, University of Peking (China)
- (R)Evolutionary Catalysis
- Prof.
Dr. C. Claver (Universitat Rovira i Virgili, Tarragona)
- Hydroformylation of alkenes; polyketone formation; asymmetric hydrogenation. - Prof. Dr. B. Chaudret (Laboratoire
de Chimie de Coordination du CNRS, lié par convention à l’Université Paul
Sabatier, Toulouse)
- Structure and reactivity of dihydrogen complexes of ruthenium and nanoparticles - Dr. C.
Bo (Universitat Rovira I Virgil, Tarragona, Spain)
- QM/MM study of bite angle effects in hydroformylation - Prof. Poliakoff (University of Nottingham)
- Catalysis in supercritical CO2 - Prof Sanders (Cambridge)
- Supramolecular porphyrin chemistry - Prof Berkessel (Universität zu Köln)
- Chiral porphyrins - Prof F. Zerbetto (University of Bologna)
- Theoretical studies on asymmetric catalysis - Prof J. Kikuchi Nara (Institute of science and technology Japan)
- Catalytically active Cerasomes - Prof R. Réau (University of Rennes)
- Supramolecular Encapsulation of phosphazole ligands
National Collaborations
- Prof. K. Lammertsma & Dr. Chris Slootweg, Vrije Universiteit Amsterdam (VU)
- Prof. T. Dingemans (TU Delft)
- Prof. Dr. D. Vogt (Eindhoven University of Technology, Schuit Institute of Catalysis, Laboratory
for Inorganic Chemistry and Catalysis)
- Hydroformylation of higher alkenes; asymmetric hydroformylation of alkenes; hydrocyanation of alkenes; hydrovinylation. - Prof.
Dr. R.J.M. Nolte / Prof A.E. Rowan (University of Nijmegen)
- Supramolecular catalysis. - Prof. G. van Koten (University of Utrecht)
- Carbosilane dendrimers as support for homogeneous catalysts. - Prof.
J.G. de Vries (University of Groningen, DSM)
- Heck reaction, allylic substitution. - Prof.
Dr. de Haan (Technical University of Twente)
- New separation methods and reactor design for homogeneous catalysis. - Prof. Dr. B. Hessen (University of Groningen)
- Polymerization - Prof. Dr. E.W. Meier, Dr. A.P.H.J. Schenning (TUE)
- Development of novel catalysts for the preparation of new photoactive polymeric materials. - Prof J. van Hest / Prof. A.E. Rowan (RU)
- Chemo-bio catalysis - Dr. R.P. Sijbesma (TUE)
- Sonor catalysis, Supramolecular catalyst immobilization - Dr. M. Bickelhaupt
- Theoretical work
Collaborations within the UvA
- Prof.
dr. C.J. Elsevier (HIMS UvA):
- Olefin polymerization - Prof. dr. H. Hiemstra, Dr. J. van Maarsseveen (HIMS UvA):
- Palladium and ruthenium catalysis and carbosilane dendrimers - Dr. Meier (UvA)
- Theoretically work - Prof L. de Cola (UvA)
- Dendrimers and supramolecular chemistry - Prof R. Wever (UvA)
- Chemo-bio catalysis - Dr. G. Rothenberg (UvA)
- Theoretically work
- http://www.science.uva.nl/~gadi/